当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysts for the Enzymatic Lipidation of Peptides
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.accounts.2c00108 Yiwu Zheng 1 , Ying Cong 2 , Eric W Schmidt 2 , Satish K Nair 1, 3, 4
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.accounts.2c00108 Yiwu Zheng 1 , Ying Cong 2 , Eric W Schmidt 2 , Satish K Nair 1, 3, 4
Affiliation
|
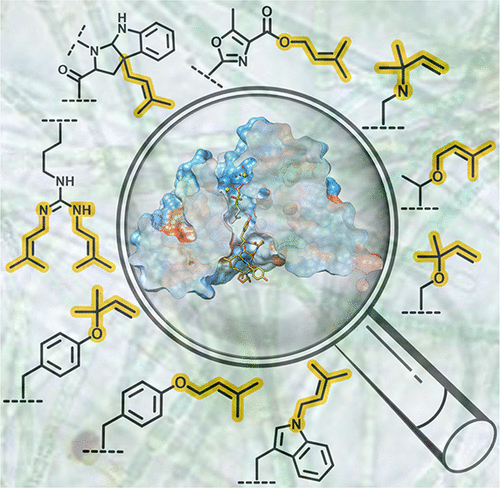
Biologically active peptides are a major growing class of drugs, but their therapeutic potential is constrained by several limitations including bioavailability and poor pharmacokinetics. The attachment of functional groups like lipids has proven to be a robust and effective strategy for improving their therapeutic potential. Biochemical and bioactivity-guided screening efforts have identified the cyanobactins as a large class of ribosomally synthesized and post-translationally modified peptides (RiPPs) that are modified with lipids. These lipids are attached by the F superfamily of peptide prenyltransferase enzymes that utilize 5-carbon (prenylation) or 10-carbon (geranylation) donors. The chemical structures of various cyanobactins initially showed isoprenoid attachments on Ser, Thr, or Tyr. Biochemical characterization of the F prenyltransferases from the corresponding clusters shows that the different enzymes have different acceptor residue specificities but are otherwise remarkably sequence tolerant. Hence, these enzymes are well suited for biotechnological applications. The crystal structure of the Tyr O-prenyltransferase PagF reveals that the F enzyme shares a domain architecture reminiscent of a canonical ABBA prenyltransferase fold but lacks secondary structural elements necessary to form an enclosed active site. Binding of either cyclic or linear peptides is sufficient to close the active site to allow for productive catalysis, explaining why these enzymes cannot use isolated amino acids as substrates.
中文翻译:

肽酶促脂化的催化剂
生物活性肽是一类不断增长的主要药物,但其治疗潜力受到多种限制,包括生物利用度和药代动力学较差。脂质等官能团的附着已被证明是提高其治疗潜力的稳健且有效的策略。生物化学和生物活性引导的筛选工作已将蓝藻素鉴定为一大类核糖体合成和翻译后修饰的肽(RiPP),并用脂质修饰。这些脂质由肽异戊烯基转移酶 F 超家族附着,该酶利用 5-碳(异戊二烯化)或 10-碳(香叶基化)供体。各种蓝菌素的化学结构最初显示出类异戊二烯附着在 Ser、Thr 或 Tyr 上。来自相应簇的 F 异戊二烯基转移酶的生化特征表明,不同的酶具有不同的受体残基特异性,但在其他方面具有显着的序列耐受性。因此,这些酶非常适合生物技术应用。 Tyr O -异戊二烯基转移酶 PagF 的晶体结构表明,F 酶具有类似于典型 ABBA 异戊二烯基转移酶折叠的结构域结构,但缺乏形成封闭活性位点所需的二级结构元件。环状或线性肽的结合足以关闭活性位点以实现高效催化,这解释了为什么这些酶不能使用分离的氨基酸作为底物。
更新日期:2022-04-20
中文翻译:

肽酶促脂化的催化剂
生物活性肽是一类不断增长的主要药物,但其治疗潜力受到多种限制,包括生物利用度和药代动力学较差。脂质等官能团的附着已被证明是提高其治疗潜力的稳健且有效的策略。生物化学和生物活性引导的筛选工作已将蓝藻素鉴定为一大类核糖体合成和翻译后修饰的肽(RiPP),并用脂质修饰。这些脂质由肽异戊烯基转移酶 F 超家族附着,该酶利用 5-碳(异戊二烯化)或 10-碳(香叶基化)供体。各种蓝菌素的化学结构最初显示出类异戊二烯附着在 Ser、Thr 或 Tyr 上。来自相应簇的 F 异戊二烯基转移酶的生化特征表明,不同的酶具有不同的受体残基特异性,但在其他方面具有显着的序列耐受性。因此,这些酶非常适合生物技术应用。 Tyr O -异戊二烯基转移酶 PagF 的晶体结构表明,F 酶具有类似于典型 ABBA 异戊二烯基转移酶折叠的结构域结构,但缺乏形成封闭活性位点所需的二级结构元件。环状或线性肽的结合足以关闭活性位点以实现高效催化,这解释了为什么这些酶不能使用分离的氨基酸作为底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号