当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Achieving Theory–Experiment Parity for Activity and Selectivity in Heterogeneous Catalysis Using Microkinetic Modeling
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.accounts.2c00058 Wenbo Xie 1 , Jiayan Xu 1 , Jianfu Chen 2 , Haifeng Wang 2 , P Hu 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.accounts.2c00058 Wenbo Xie 1 , Jiayan Xu 1 , Jianfu Chen 2 , Haifeng Wang 2 , P Hu 1, 2
Affiliation
|
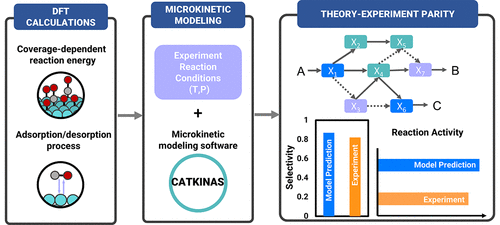
Microkinetic modeling based on density functional theory (DFT) energies plays an essential role in heterogeneous catalysis because it reveals the fundamental chemistry for catalytic reactions and bridges the microscopic understanding from theoretical calculations and experimental observations. Microkinetic modeling requires building a set of ordinary differential equations (ODEs) based on the calculation results of thermodynamic properties of adsorbates and kinetic parameters for the reaction elementary steps. Solving a microkinetic model can extract information on catalytic chemistry, including critical reaction intermediates, reaction pathways, the surface species distribution, activity, and selectivity, thus providing vital guidelines for altering catalysts.
中文翻译:

使用微动力学模型实现多相催化中活性和选择性的理论-实验均等
基于密度泛函理论 (DFT) 能量的微动力学建模在非均相催化中发挥着重要作用,因为它揭示了催化反应的基本化学过程,并在理论计算和实验观察中架起了微观理解的桥梁。微动力学建模需要根据吸附物的热力学性质和反应基本步骤的动力学参数的计算结果,建立一组常微分方程 (ODE)。求解微动力学模型可以提取有关催化化学的信息,包括关键反应中间体、反应途径、表面物种分布、活性和选择性,从而为改变催化剂提供重要指导。
更新日期:2022-04-20
中文翻译:

使用微动力学模型实现多相催化中活性和选择性的理论-实验均等
基于密度泛函理论 (DFT) 能量的微动力学建模在非均相催化中发挥着重要作用,因为它揭示了催化反应的基本化学过程,并在理论计算和实验观察中架起了微观理解的桥梁。微动力学建模需要根据吸附物的热力学性质和反应基本步骤的动力学参数的计算结果,建立一组常微分方程 (ODE)。求解微动力学模型可以提取有关催化化学的信息,包括关键反应中间体、反应途径、表面物种分布、活性和选择性,从而为改变催化剂提供重要指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号