Journal of Advanced Research ( IF 10.7 ) Pub Date : 2022-04-15 , DOI: 10.1016/j.jare.2022.04.006 Congcong Yan 1 , Ke Li 1 , Fanling Meng 2 , Lu Chen 1 , Jingting Zhao 1 , Zicheng Zhang 1 , Dandan Xu 3 , Jie Sun 1 , Meng Zhou 1
|
Introduction
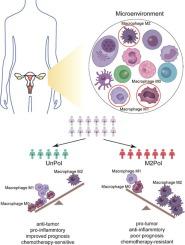
Increasing evidence demonstrates that the activation states and diverse spectrum of macrophage subtypes display dynamic heterogeneity in the tumor microenvironment, which plays a critical role in a variety of cancer types.
Objectives
To investigate the heterogeneity and the homeostasis of different macrophage subtypes, as well as their effect on biological and clinical manifestations of ovarian cancer (OV).
Method
Integrated immunogenomic analysis of single-cell and bulk tissue transcriptome profiling was performed to systematically investigate the association between macrophage activation and prognostic and therapeutic efficacy. Consensus clustering analysis was used to define novel macrophage subtypes. An artificial neural network was used to simulate the dynamic activation of macrophages.
Results
The pan-cohort results suggested that high relative infiltration abundance of M0 and M1 macrophages was associated with improved outcome and therapeutic efficacy. However, it was the opposite for M2 macrophages. Unsupervised consensus clustering analysis revealed two OV subgroups characterized by a balance between M0, M1 and M2 macrophages with distinct clinical and immunological behaviors. Finally, a macrophage polarization-derived artificial neural network model was proposed to serve as a robust prognostic factor and predictive biomarker for therapeutic efficacy, which was validated in different independent patient cohorts.
Conclusion
The present study provides a new understanding of macrophage heterogeneity and its association with OV prognosis and underlines the future clinical potential of a macrophage activation model for tumor prevention and treatment.
中文翻译:

单细胞和大量组织转录组分析的综合免疫基因组学分析揭示了与卵巢癌免疫学和临床不同行为相关的巨噬细胞激活范例
介绍
越来越多的证据表明,巨噬细胞亚型的激活状态和不同谱系在肿瘤微环境中表现出动态异质性,这在多种癌症类型中起着至关重要的作用。
目标
研究不同巨噬细胞亚型的异质性和稳态,以及它们对卵巢癌 (OV) 生物学和临床表现的影响。
方法
对单细胞和大量组织 转录组分析进行了综合免疫基因组分析,以系统地研究巨噬细胞活化与预后和治疗效果之间的关联。共识聚类分析用于定义新的巨噬细胞亚型。人工神经网络被用来模拟巨噬细胞的动态激活。
结果
泛队列结果表明,M0 和 M1 巨噬细胞的高相对浸润丰度与改善的结果和治疗效果相关。然而,对于 M2 巨噬细胞则相反。无监督共识聚类分析揭示了两个 OV 亚组,其特征在于 M0、M1 和 M2 巨噬细胞之间的平衡,具有不同的临床和免疫学行为。最后,提出了巨噬细胞极化衍生的人工神经网络模型作为稳健的预后因素和治疗效果的预测生物标志物,并在不同的独立患者队列中得到验证。
结论
本研究提供了对巨噬细胞异质性及其与 OV 预后关联的新认识,并强调了巨噬细胞激活模型在肿瘤预防和治疗中的未来临床潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号