当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the highest oxidation states of the actinoids in AnO4 molecules (An = Ac – Cm): A DMRG-CASSCF study
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-04-14 , DOI: 10.1002/jcc.26856 Jun-Bo Lu 1, 2 , Xue-Lian Jiang 1 , Jia-Qi Wang 2 , Han-Shi Hu 2 , W H Eugen Schwarz 2, 3 , Jun Li 1, 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-04-14 , DOI: 10.1002/jcc.26856 Jun-Bo Lu 1, 2 , Xue-Lian Jiang 1 , Jia-Qi Wang 2 , Han-Shi Hu 2 , W H Eugen Schwarz 2, 3 , Jun Li 1, 2
Affiliation
|
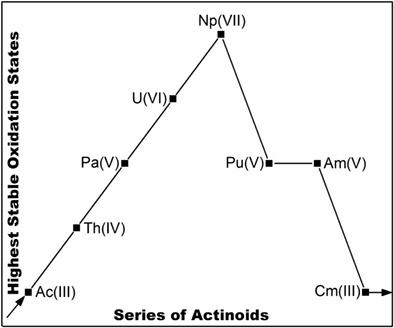
Actinoid tetroxide molecules AnO4 (An = Ac – Cm) are investigated with the ab initio density matrix renormalization group (DMRG) approach. Natural orbital shapes are used to read out the oxidation state (OS) of the f-elements, and the atomic orbital energies and radii are used to explain the trends. The highest OSs reveal a “volcano”-type variation: For An = Ac – Np, the OSs are equal to the number of available valence electrons, that is, AcIII, ThIV, PaV, UVI, and NpVII. Starting with plutonium as the turning point, the highest OSs in the most stable AnO4 isomers then decrease as PuV, AmV, and CmIII, indicating that the 5f-electrons are hard to be fully oxidized off from Pu onward. The variations are related to the actinoid contraction and to the 5f-covalency characteristics. Combined with previous work on OSs, we review their general trends throughout the periodic table, providing fundamental understanding of OS-relevant phenomena.
中文翻译:

关于 AnO4 分子中锕系元素的最高氧化态 (An = Ac – Cm):DMRG-CASSCF 研究
使用从头算密度矩阵重整化组 (DMRG) 方法研究四氧化锕类分子 AnO 4 (An = Ac – Cm)。自然轨道形状用于读出 f 元素的氧化态 (OS),原子轨道能量和半径用于解释趋势。最高的 OS 显示出“火山”型变化:对于 An = Ac – Np,OS 等于可用价电子的数量,即 Ac III、Th IV、Pa V、U VI和 Np VII。以钚作为转折点开始,最稳定的 AnO 4异构体中的最高 OS 随 Pu V、Am V和 Cm而降低III,表明 5f 电子很难从 Pu 开始完全氧化掉。这些变化与锕系收缩和 5f-共价特性有关。结合之前关于操作系统的工作,我们回顾了它们在整个元素周期表中的一般趋势,提供了对操作系统相关现象的基本理解。
更新日期:2022-04-14
中文翻译:

关于 AnO4 分子中锕系元素的最高氧化态 (An = Ac – Cm):DMRG-CASSCF 研究
使用从头算密度矩阵重整化组 (DMRG) 方法研究四氧化锕类分子 AnO 4 (An = Ac – Cm)。自然轨道形状用于读出 f 元素的氧化态 (OS),原子轨道能量和半径用于解释趋势。最高的 OS 显示出“火山”型变化:对于 An = Ac – Np,OS 等于可用价电子的数量,即 Ac III、Th IV、Pa V、U VI和 Np VII。以钚作为转折点开始,最稳定的 AnO 4异构体中的最高 OS 随 Pu V、Am V和 Cm而降低III,表明 5f 电子很难从 Pu 开始完全氧化掉。这些变化与锕系收缩和 5f-共价特性有关。结合之前关于操作系统的工作,我们回顾了它们在整个元素周期表中的一般趋势,提供了对操作系统相关现象的基本理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号