Structure ( IF 4.4 ) Pub Date : 2022-04-08 , DOI: 10.1016/j.str.2022.03.005 Chandrima Jash 1 , Akiva Feintuch 1 , Shira Nudelman 1 , Nurit Manukovsky 1 , Elwy H Abdelkader 2 , Sudeshna Bhattacharya 1 , Gunnar Jeschke 3 , Gottfried Otting 2 , Daniella Goldfarb 1
|
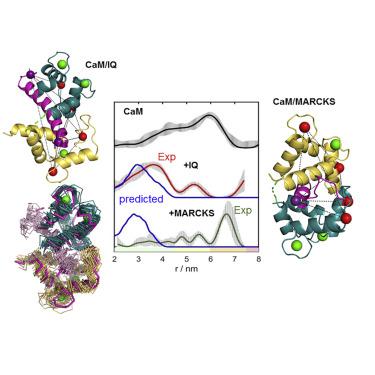
Calmodulin (CaM) is a calcium-binding protein that regulates the function of many proteins by indirectly conferring Ca2+ sensitivity, and it undergoes a large conformational change on partners' binding. We compared the solution binding mode of the target peptides MARCKS and IQ by double electron-electron resonance (DEER) distance measurements and paramagnetic NMR. We combined nitroxide and Gd(III) spin labels, including specific substitution of one of the Ca2+ ions in the CaM mutant N60D by a Gd(III) ion. The binding of MARCKS to holo-CaM resulted neither in a closed conformation nor in a unique relative orientation between the two CaM domains, in contrast with the crystal structure. Binding of IQ to holo-CaM did generate a closed conformation. Using elastic network modeling and 12 distance restraints obtained from multiple holo-CaM/IQ DEER data, we derived a model of the solution structure, which is in reasonable agreement with the crystal structure.
中文翻译:

DEER 实验揭示了溶液中具有 IQ 和 MARCKS 肽的钙调蛋白复合物之间的根本差异
钙调蛋白 (CaM) 是一种钙结合蛋白,通过间接赋予 Ca 2+敏感性来调节许多蛋白质的功能,并且它对伴侣的结合经历了大的构象变化。我们通过双电子-电子共振 (DEER) 距离测量和顺磁 NMR 比较了目标肽 MARCKS 和 IQ 的溶液结合模式。我们结合了氮氧化物和 Gd(III) 自旋标签,包括用 Gd(III) 离子特异性取代 CaM 突变体 N60D 中的一种 Ca 2+离子。与晶体结构相比,MARCKS 与全息-CaM 的结合既不会导致闭合构象,也不会导致两个 CaM 结构域之间的独特相对方向。智商与全息的绑定-CaM 确实产生了闭合构象。利用弹性网络建模和从多个全息-CaM/IQ DEER数据中获得的12个距离约束,我们推导出了与晶体结构合理吻合的溶液结构模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号