Structure ( IF 4.4 ) Pub Date : 2022-04-04 , DOI: 10.1016/j.str.2022.03.008 Zhen-Lu Li 1 , Carla Mattos 2 , Matthias Buck 3
|
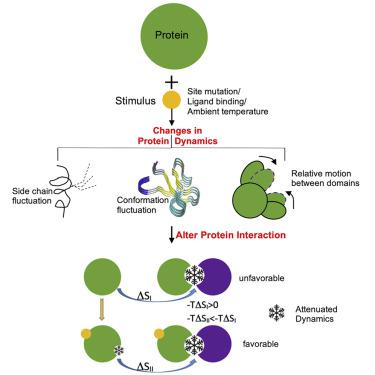
Dynamic allostery emphasizes a role of entropy change manifested as a sole change in protein fluctuations without structural changes. This kind of entropy-driven effect remains largely understudied. The most significant examples involve protein-ligand interactions, leaving protein-protein interactions, which are critical in signaling and other cellular events, largely unexplored. Here we study an example of how protein-protein interaction (binding of Ras to the Ras binding domain [RBD] of the effector protein Raf) affects a subsequent protein association process (Ras dimerization) by quenching Ras internal motions through dynamic allostery. We also investigate the influence of point mutations or ambient temperature, respectively, on the protein dynamics and interaction of two other systems: in adenylate kinase (ADK) and in the EphA2 SAM:Ship2 SAM complex. Based on these examples, we postulate that there are different ways in which dynamic-change-driven protein interactions are manifested and that it is likely a general biological phenomenon.
中文翻译:

动态变化驱动蛋白质相互作用原理的计算研究
动态变构强调熵变化的作用,表现为蛋白质波动的唯一变化而没有结构变化。这种熵驱动的效应在很大程度上仍未得到充分研究。最重要的例子涉及蛋白质 - 配体相互作用,而蛋白质 - 蛋白质相互作用,在信号传导和其他细胞事件中至关重要,在很大程度上未被探索。在这里,我们研究了蛋白质-蛋白质相互作用(Ras 与效应蛋白 Raf 的 Ras 结合域 [RBD] 的结合)如何通过动态变构抑制 Ras 内部运动来影响后续蛋白质结合过程(Ras 二聚化)的示例。我们还分别研究了点突变或环境温度对蛋白质动力学和其他两个系统相互作用的影响:在腺苷酸激酶 (ADK) 和 EphA2 SAM 中:Ship2 SAM 复合体。基于这些例子,我们假设动态变化驱动的蛋白质相互作用有不同的表现方式,这可能是一种普遍的生物学现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号