当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis acid-catalyzed [4 + 2] Cycloaddition of 3-Alkyl-2-vinylindoles with β,γ-unsaturated α-ketoesters
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-2-1 , DOI: 10.1016/j.gresc.2021.11.003 Yuwen Sun , Zhaoshan Wang , Shufang Wu , Yuchen Zhang , Feng Shi
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-2-1 , DOI: 10.1016/j.gresc.2021.11.003 Yuwen Sun , Zhaoshan Wang , Shufang Wu , Yuchen Zhang , Feng Shi
|
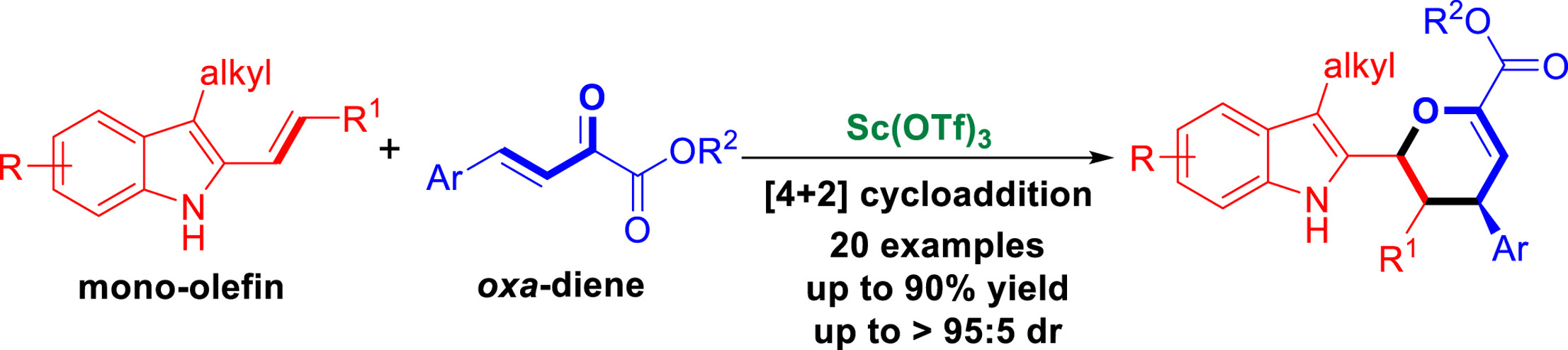
A Lewis acid-catalyzed [4 ?+ ?2] cycloaddition of 3-alkyl-2-vinylindoles with β,γ-unsaturated α-ketoesters has been established in the presence of Sc(OTf)3, which afforded a series of indole-containing pyran derivatives in generally good yields (up to 90% yield) with excellent diastereoselectivities (up to ?> 95:5 dr) under mild conditions. This approach not only enriches the chemistry of 3-alkyl-2-vinylindoles, but also has provided an atom-economic method for the synthesis of indole-containing pyran derivatives with potential bioactivity.
中文翻译:

路易斯酸催化的 [4 + 2] 3-Alkyl-2-vinylindoles 与 β,γ-不饱和 α-酮酯的环加成
已经在 Sc(OTf)3 存在下建立了路易斯酸催化的 3-烷基-2-乙烯基吲哚与 β,γ-不饱和 α-酮酯的 [4 ?+ ?2] 环加成反应,得到了一系列吲哚-含有吡喃衍生物,在温和条件下通常具有良好的收率(高达 90% 的收率)和出色的非对映选择性(高达 ?> 95:5 dr)。该方法不仅丰富了3-烷基-2-乙烯基吲哚的化学性质,而且为合成具有潜在生物活性的含吲哚吡喃衍生物提供了原子经济方法。
更新日期:2022-03-28
中文翻译:

路易斯酸催化的 [4 + 2] 3-Alkyl-2-vinylindoles 与 β,γ-不饱和 α-酮酯的环加成
已经在 Sc(OTf)3 存在下建立了路易斯酸催化的 3-烷基-2-乙烯基吲哚与 β,γ-不饱和 α-酮酯的 [4 ?+ ?2] 环加成反应,得到了一系列吲哚-含有吡喃衍生物,在温和条件下通常具有良好的收率(高达 90% 的收率)和出色的非对映选择性(高达 ?> 95:5 dr)。该方法不仅丰富了3-烷基-2-乙烯基吲哚的化学性质,而且为合成具有潜在生物活性的含吲哚吡喃衍生物提供了原子经济方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号