Journal of Advanced Research ( IF 11.4 ) Pub Date : 2022-03-04 , DOI: 10.1016/j.jare.2022.03.001 Chang Xue 1 , Huimin Niu 2 , Shuyao Hu 1 , Zhe Yang 1 , Lei Wang 3 , Zai-Sheng Wu 1
|
Introduction
MicroRNAs (miRNAs) have been revealed to be critical genetic regulators in various physiological processes and thus quantitative information on the expression level of critical miRNAs has important implications for the initiation and development of human diseases, including cancers.
Objectives
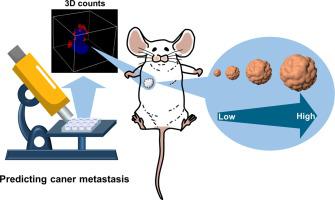
We herein develop three-dimensionally (3D) counting of intracellular fluorescent spots for accurately evaluating microRNA-21 (miRNA-21) expression in individual HeLa cells based on stimuli-activated in situ growth of optical DNA flares, grid-patterned DNA-protein hybrids (GDPHs).
Methods
Target miRNA is sequence-specifically detected down to 10 pM owing to efficient signal amplification. Within living cells, GDPH flares are nuclease resistant and discrete objects with retarded mobility, enabling the screening of intracellular location and distribution of miRNAs and realizing in situ counting of target species with a high accuracy.
Results
The quantitative results of intracellular miRNAs by 3D fluorescence counts are consistent with qPCR gold standard assay, exhibiting the superiority over 2D counts. By screening the expression of intracellular miR-21 that can down-regulate the programmed cell death 4 (PDCD4) protein, the proliferation and migration of HeLa cells, including artificially-regulated ones, were well estimated, thus enabling the prediction of cancer metastasis in murine tumor models.
Conclusion
The experiments in vitro, ex vivo and in vivo demonstrate that GDPH-based 3D fluorescence counts at the single cell level provide a valuable molecular tool for understanding biological function of miRNAs and especially for recognizing aggressive CTCs, offering a design blueprint for further expansion of DNA structural nanotechnology in predicting distant metastasis and prevention of tumor recurrence after primary resection.
中文翻译:

通过基于 DNA 耀斑原位生长的荧光点细胞内 3D 计数视觉预测 microRNA 调节的肿瘤转移
介绍
MicroRNA (miRNA) 已被证明是各种生理过程中的关键遗传调节剂,因此关于关键 miRNA 表达水平的定量信息对人类疾病(包括癌症)的发生和发展具有重要意义。
目标
我们在此开发了细胞内荧光点的三维 (3D) 计数,以根据光学 DNA 耀斑、网格图案 DNA-蛋白质杂交体的刺激激活原位生长,准确评估单个 HeLa 细胞中的 microRNA-21 (miRNA-21) 表达(GDPH)。
方法
由于有效的信号放大,目标 miRNA 可以序列特异性地检测到低至 10 pM。在活细胞内,GDPH 耀斑是一种耐核酸酶的离散物体,具有迟缓的运动能力,能够筛选 miRNA 在细胞内的定位和分布,实现目标物种的高精度原位计数。
结果
3D 荧光计数的细胞内 miRNA 定量结果与 qPCR 金标准测定一致,表现出优于 2D 计数的优势。通过筛选可下调程序性细胞死亡4(PDCD4)蛋白的细胞内miR-21的表达,很好地估计了HeLa细胞(包括人工调节的HeLa细胞)的增殖和迁移,从而能够预测癌症转移。小鼠肿瘤模型。
结论
体外、离体和体内实验表明,基于 GDPH 的单细胞水平 3D 荧光计数为了解 miRNA 的生物学功能,尤其是识别侵袭性 CTC 提供了有价值的分子工具,为进一步扩展 DNA 提供了设计蓝图结构纳米技术预测远处转移和预防初次切除后肿瘤复发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号