Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molten salt synthesis of carbon-supported Pt–rare earth metal nanoalloy catalysts for oxygen reduction reaction
RSC Advances ( IF 3.9 ) Pub Date : 2022-02-09 , DOI: 10.1039/d1ra09400a Yulin Jiang 1 , Tao Fu 2 , Jiaxiang Liu 3 , Jinbao Zhao 1, 3 , Bing Li 2, 4 , Zhenjie Chen 1
RSC Advances ( IF 3.9 ) Pub Date : 2022-02-09 , DOI: 10.1039/d1ra09400a Yulin Jiang 1 , Tao Fu 2 , Jiaxiang Liu 3 , Jinbao Zhao 1, 3 , Bing Li 2, 4 , Zhenjie Chen 1
Affiliation
|
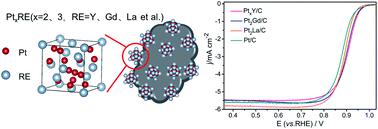
The synthesis of nano-sized alloys of Pt and rare earth (RE) metal catalysts has been a huge challenge due to a significantly large standard reduction potential difference of Pt and RE metals and the high synthesis temperature. PtxY/C catalysts with an average particle size of around 21 nm, were synthesized by mixing K2PtCl4 with Y2O3 (a molar ratio of Pt : Y = 1 : 1) with a carbon support in a molten LiCl–CaH2 system by a one-step molten salt synthesis method at 600 °C. The synthesis processes of the PtxY/C alloys are proposed as follows: Pt nanoparticles were first obtained by the reaction of K2PtCl4 and CaH2 at 210 °C, then Y ions were preferentially reduced on the Pt nanoparticle surface by the reduction of CaH2, followed by PtxY alloy formation in the molten LiCl–CaH2 system at 600 °C. Molten LiCl provides a strong reducing environment and lowers the formation temperature of alloys. Pt2Gd/C and Pt2La/C were also obtained with Gd2O3 and La2O3 as the starting raw materials, respectively by using the same process. When investigated as an electrocatalyst for the oxygen reduction reaction (ORR), the half-wave potentials of PtxRE/Cs are all more positive than that of commercial Pt/C catalyst (e.g., 0.905 V for PtxY/C while 0.880 V for JM Pt/C), and the nano-sized PtxY/C alloy shows higher electrocatalytic activity toward the ORR and preferable catalytic durability with respect to JM Pt/C catalysts. This facile synthesis method provides an effective strategy for the preparation of Pt–RE based multicomponent nanoalloys, especially in large-scale production.
中文翻译:

用于氧还原反应的碳负载铂稀土金属纳米合金催化剂的熔盐合成
由于 Pt 和稀土金属的标准还原电位差显着大以及合成温度高,Pt 和稀土 (RE) 金属催化剂的纳米合金的合成一直是一个巨大的挑战。通过将 K 2 PtCl 4与 Y 2 O 3(Pt : Y 的摩尔比 = 1 : 1)与碳载体在熔融的 LiCl 中混合来合成平均粒径约为 21 nm 的Pt x Y/C 催化剂-CaH 2体系,采用一步熔盐合成法,600℃。Pt x Y/C合金的合成工艺如下:首先通过K 2 PtCl 4反应获得Pt纳米颗粒和 CaH 2在 210 ℃,然后 Y 离子通过 CaH 2 的还原优先在 Pt 纳米颗粒表面上被还原,然后在 600 ℃的熔融 LiCl-CaH 2体系中形成Pt x Y 合金。熔融的 LiCl 提供了强大的还原环境并降低了合金的形成温度。Pt 2 Gd/C 和Pt 2 La/C 也分别以Gd 2 O 3和La 2 O 3为起始原料,通过相同的工艺得到。当作为氧还原反应 (ORR) 的电催化剂进行研究时,Pt x的半波电位RE/Cs 都比商业 Pt/C 催化剂更积极(例如,Pt x Y/C为 0.905 V ,JM Pt/C 为 0.880 V),纳米尺寸的 Pt x Y/C 合金表现出更高的电催化对 ORR 的活性和相对于 JM Pt/C 催化剂的优选催化耐久性。这种简便的合成方法为制备 Pt-RE 基多组分纳米合金提供了一种有效的策略,特别是在大规模生产中。
更新日期:2022-02-09
中文翻译:

用于氧还原反应的碳负载铂稀土金属纳米合金催化剂的熔盐合成
由于 Pt 和稀土金属的标准还原电位差显着大以及合成温度高,Pt 和稀土 (RE) 金属催化剂的纳米合金的合成一直是一个巨大的挑战。通过将 K 2 PtCl 4与 Y 2 O 3(Pt : Y 的摩尔比 = 1 : 1)与碳载体在熔融的 LiCl 中混合来合成平均粒径约为 21 nm 的Pt x Y/C 催化剂-CaH 2体系,采用一步熔盐合成法,600℃。Pt x Y/C合金的合成工艺如下:首先通过K 2 PtCl 4反应获得Pt纳米颗粒和 CaH 2在 210 ℃,然后 Y 离子通过 CaH 2 的还原优先在 Pt 纳米颗粒表面上被还原,然后在 600 ℃的熔融 LiCl-CaH 2体系中形成Pt x Y 合金。熔融的 LiCl 提供了强大的还原环境并降低了合金的形成温度。Pt 2 Gd/C 和Pt 2 La/C 也分别以Gd 2 O 3和La 2 O 3为起始原料,通过相同的工艺得到。当作为氧还原反应 (ORR) 的电催化剂进行研究时,Pt x的半波电位RE/Cs 都比商业 Pt/C 催化剂更积极(例如,Pt x Y/C为 0.905 V ,JM Pt/C 为 0.880 V),纳米尺寸的 Pt x Y/C 合金表现出更高的电催化对 ORR 的活性和相对于 JM Pt/C 催化剂的优选催化耐久性。这种简便的合成方法为制备 Pt-RE 基多组分纳米合金提供了一种有效的策略,特别是在大规模生产中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号