Cell Chemical Biology ( IF 6.6 ) Pub Date : 2022-01-25 , DOI: 10.1016/j.chembiol.2022.01.004 Chia-Hung Christine Hsiao 1 , Khiem Nguyen 1 , Yiming Jin 1 , Olga Vinogradova 1 , Andrew J Wiemer 2
|
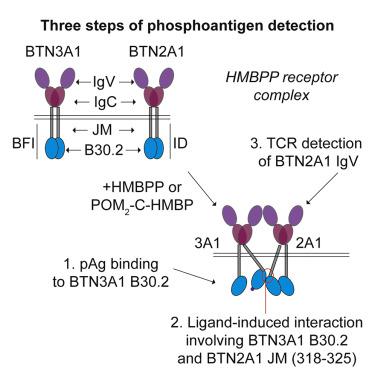
The ligand-bound (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) receptor (BTN3A1 and BTN2A1) is detectable by the T cell receptor (TCR) of Vγ9Vδ2 T cells. Although BTN3A1 binds to phosphoantigens (pAgs), the mechanisms resulting in receptor activation are not clear. We used CRISPR-Cas9, ELISA, nano-bioluminescence resonance energy transfer (BRET), and isothermal titration calorimetry (ITC) to evaluate the role of BTN2A1. Depletion of BTN2A1 and rescue experiments demonstrate that its internal domain is essential for pAg detection. Internal hetero-BRET signals are observed between BTN2A1 and BTN3A1 that are increased by pAg. ITC detects a direct interaction between the intracellular domains of BTN3A1 and BTN2A1 only in the presence of pAg. This interaction is abrogated by removal of the BTN2A1 juxtamembrane (JM) region but not by removal of the BTN3A1 JM region. Regional mutations between BTN2A1 316–326 clearly affect the interferon γ (IFNγ) response and hetero-BRET signal. Mutations to amino acids L318, W320, and L325 indicate that these amino acids are crucial. This study demonstrates a pAg-inducible interaction between BTN2A1 and BTN3A1 internal domains.
中文翻译:

HMBPP 受体复合物中嗜乳脂蛋白 2A1 和 3A1 内部结构域之间配体诱导的相互作用
配体结合的 ( E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) 受体(BTN3A1 和 BTN2A1)可被 Vγ9Vδ2 T 细胞的 T 细胞受体 (TCR) 检测到。尽管 BTN3A1 与磷酸抗原 (pAg) 结合,但导致受体激活的机制尚不清楚。我们使用 CRISPR-Cas9、ELISA、纳米生物发光共振能量转移 (BRET) 和等温滴定量热法 (ITC) 来评估 BTN2A1 的作用。BTN2A1 的消耗和拯救实验证明其内部结构域对于 pAg 检测至关重要。在 BTN2A1 和 BTN3A1 之间观察到由 pAg 增加的内部异 BRET 信号。ITC 仅在存在 pAg 的情况下检测到 BTN3A1 和 BTN2A1 的细胞内结构域之间的直接相互作用。这种相互作用是通过去除 BTN2A1 近膜 (JM) 区域而不是通过去除 BTN3A1 JM 区域来消除的。BTN2A1 316–326 之间的区域突变明显影响干扰素 γ (IFNγ) 反应和异源 BRET 信号。氨基酸 L318、W320 和 L325 的突变表明这些氨基酸至关重要。该研究证明了 BTN2A1 和 BTN3A1 内部域之间的 pAg 诱导相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号