当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Analysis of CO2 Hydrogenation to Long-Chain Hydrocarbons on a Supported Iron Catalyst
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.iecr.1c04018 Lucas Brübach 1 , Daniel Hodonj 1 , Peter Pfeifer 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.iecr.1c04018 Lucas Brübach 1 , Daniel Hodonj 1 , Peter Pfeifer 1
Affiliation
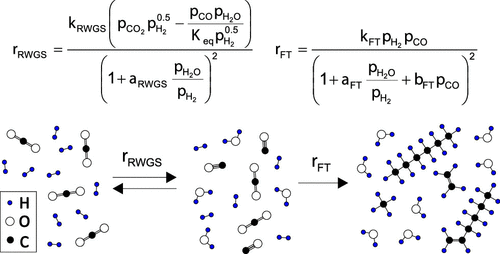
|
Hydrogenation of CO2 to long-chain hydrocarbons via combined reverse water gas shift (RWGS) and Fischer–Tropsch (FT) gained much attention in the last years as a way to produce sustainable hydrocarbons for the chemical industry or fuel applications. Despite the large amount of interest in the reaction, so far only a few studies have been conducted regarding the kinetics. In this study we carefully investigated the kinetics of an alumina supported iron catalyst at 280–320 °C, 10–20 bar, 900–120 000 mLN h–1 g–1, and a H2/CO2 molar inlet ratio of 2–4. Special attention was focused toward the thermodynamic constraints under reaction conditions. Based on elementary reaction steps according to recent mechanistic investigations, we derived new Langmuir–Hinshelwood–Hougen–Watson type kinetic expressions which allow an excellent reproduction of the experimental data and outperform existent models. Possible model combinations were discriminated against each other, and the best fit was obtained for the assumption of H-assisted CO2 and H-assisted CO dissociation mechanisms for RWGS and FT, respectively. Model uncertainties that are introduced by the RWGS being close to equilibrium are discussed in detail and are possibly a reason for strongly varying results for activation energies between different studies. The detrimental effect of water vapor on the reaction progression is analyzed numerically and can be attributed to two parameters: kinetic inhibition via strong adsorption of oxygen containing species and thermodynamic constraints by shifting the equilibrium CO partial pressures to lower values.
中文翻译:

负载型铁催化剂上 CO2 加氢生成长链烃的动力学分析
通过组合的反向水煤气变换 (RWGS) 和费托 (FT)将 CO 2加氢为长链碳氢化合物在过去几年中作为一种为化学工业或燃料应用生产可持续碳氢化合物的方法引起了广泛关注。尽管人们对该反应很感兴趣,但迄今为止只有少数关于动力学的研究。在这项研究中,我们仔细研究了氧化铝负载的铁催化剂在 280–320 °C、10–20 bar、900–120 000 mL N h –1 g –1和 H 2 /CO 2条件下的动力学。摩尔入口比为 2-4。特别关注反应条件下的热力学约束。基于根据最近的机理研究的基本反应步骤,我们推导出了新的 Langmuir-Hinshelwood-Hougen-Watson 型动力学表达式,它可以很好地再现实验数据并优于现有模型。可能的模型组合相互区分,并获得了 H 辅助 CO 2假设的最佳拟合分别用于 RWGS 和 FT 的 H 辅助 CO 解离机制。详细讨论了由接近平衡的 RWGS 引入的模型不确定性,这可能是不同研究之间活化能结果差异很大的原因。通过数值分析水蒸气对反应进程的不利影响,可归因于两个参数:通过强吸附含氧物质的动力学抑制和通过将平衡 CO 分压转移到较低值的热力学约束。
更新日期:2022-02-02
中文翻译:

负载型铁催化剂上 CO2 加氢生成长链烃的动力学分析
通过组合的反向水煤气变换 (RWGS) 和费托 (FT)将 CO 2加氢为长链碳氢化合物在过去几年中作为一种为化学工业或燃料应用生产可持续碳氢化合物的方法引起了广泛关注。尽管人们对该反应很感兴趣,但迄今为止只有少数关于动力学的研究。在这项研究中,我们仔细研究了氧化铝负载的铁催化剂在 280–320 °C、10–20 bar、900–120 000 mL N h –1 g –1和 H 2 /CO 2条件下的动力学。摩尔入口比为 2-4。特别关注反应条件下的热力学约束。基于根据最近的机理研究的基本反应步骤,我们推导出了新的 Langmuir-Hinshelwood-Hougen-Watson 型动力学表达式,它可以很好地再现实验数据并优于现有模型。可能的模型组合相互区分,并获得了 H 辅助 CO 2假设的最佳拟合分别用于 RWGS 和 FT 的 H 辅助 CO 解离机制。详细讨论了由接近平衡的 RWGS 引入的模型不确定性,这可能是不同研究之间活化能结果差异很大的原因。通过数值分析水蒸气对反应进程的不利影响,可归因于两个参数:通过强吸附含氧物质的动力学抑制和通过将平衡 CO 分压转移到较低值的热力学约束。











































 京公网安备 11010802027423号
京公网安备 11010802027423号