当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Correlation Analysis of 5-Chloropyrazin-2-Amine in Several Mixed Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jced.1c00515 Guangyu Xu 1 , Fanyuan Zhang 1 , Zhenghui Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jced.1c00515 Guangyu Xu 1 , Fanyuan Zhang 1 , Zhenghui Li 1
Affiliation
|
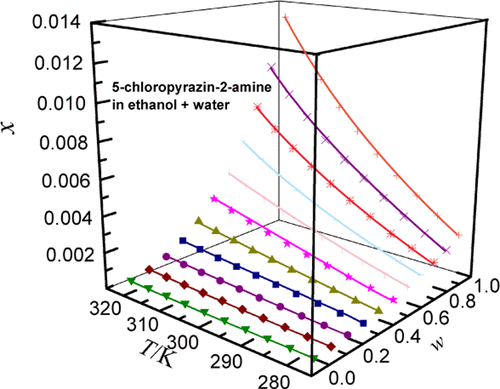
In this study, the solubility of 5-chloropyrazin-2-amine in four aqueous solutions (methanol, ethanol, n-propanol, and ethylene glycol (EG)) plus water was determined by a gravimetric method at temperatures ranging from T = 278.15 to 323.15 K at 101.2 kPa pressure. Experimental results indicated that 5-chloropyrazin-2-amine solubility positively increased with increasing temperature at a given solvent composition and decreased with increasing mass fraction of water in each system. The order of solubility from high to low on the mole fraction scale was as follows: methanol (0.02007, 323.15 K) > ethanol (0.01308, 323.15 K) > n-propanol (3.827 × 10–3, 323.15 K) > water (1.569 × 10–3, 323.15 K). Similarly, the solubility of 5-chloropyrazin-2-amine was higher in (methanol + water) than in the other three cosolvent mixtures. The solid phase crystal of 5-chloropyrazin-2-amine was tested by X-ray powder diffraction (XPRD) and the results showed no polymorphic transformation, solvate formation, or crystal transition during entire experiments. The experimental solubility values of 5-chloropyrazin-2-amine were correlated by three thermodynamic models. The results show that the largest relative average deviation (RAD) and root mean square deviation (RMSD) data were no more than 5.38% and 2.34 × 10–4, respectively. The Jouyban–Acree model gave the best correlation result in this study.
中文翻译:

5-氯吡嗪-2-胺在几种混合溶剂中的溶解度测定及相关性分析
在这项研究中,通过重量法在T = 278.15至在 101.2 kPa 压力下为 323.15 K。实验结果表明,在给定的溶剂组成下,5-氯吡嗪-2-胺的溶解度随着温度的升高而正增加,而随着每个系统中水的质量分数的增加而降低。在摩尔分数尺度上溶解度从高到低的顺序如下:甲醇(0.02007, 323.15 K)> 乙醇(0.01308, 323.15 K)>正丙醇(3.827 × 10 –3 , 323.15 K)> 水(1.569 × 10 –3, 323.15 K)。类似地,5-氯吡嗪-2-胺在(甲醇+水)中的溶解度高于在其他三种共溶剂混合物中的溶解度。通过X射线粉末衍射(XPRD)测试了5-氯吡嗪-2-胺的固相晶体,结果表明在整个实验过程中没有多晶型转变、溶剂化物形成或晶体转变。5-chloropyrazin-2-amine 的实验溶解度值通过三个热力学模型相互关联。结果表明,最大相对平均偏差 (RAD) 和均方根偏差 (RMSD) 数据分别不超过 5.38% 和 2.34 × 10 –4。Jouyban-Acree 模型在本研究中给出了最好的相关性结果。
更新日期:2022-02-10
中文翻译:

5-氯吡嗪-2-胺在几种混合溶剂中的溶解度测定及相关性分析
在这项研究中,通过重量法在T = 278.15至在 101.2 kPa 压力下为 323.15 K。实验结果表明,在给定的溶剂组成下,5-氯吡嗪-2-胺的溶解度随着温度的升高而正增加,而随着每个系统中水的质量分数的增加而降低。在摩尔分数尺度上溶解度从高到低的顺序如下:甲醇(0.02007, 323.15 K)> 乙醇(0.01308, 323.15 K)>正丙醇(3.827 × 10 –3 , 323.15 K)> 水(1.569 × 10 –3, 323.15 K)。类似地,5-氯吡嗪-2-胺在(甲醇+水)中的溶解度高于在其他三种共溶剂混合物中的溶解度。通过X射线粉末衍射(XPRD)测试了5-氯吡嗪-2-胺的固相晶体,结果表明在整个实验过程中没有多晶型转变、溶剂化物形成或晶体转变。5-chloropyrazin-2-amine 的实验溶解度值通过三个热力学模型相互关联。结果表明,最大相对平均偏差 (RAD) 和均方根偏差 (RMSD) 数据分别不超过 5.38% 和 2.34 × 10 –4。Jouyban-Acree 模型在本研究中给出了最好的相关性结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号