Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2022-01-19 , DOI: 10.1016/j.jece.2022.107225 Irem Firtina-Ertis 1
|
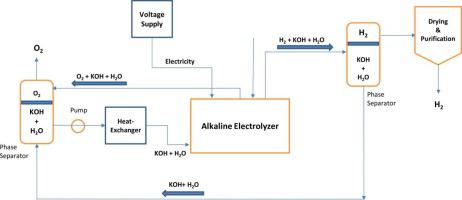
In the study, the alkaline electrolyzer is thermodynamically and electrochemically analyzed under various operating parameters for optimum hydrogen production. The effects of varying operating parameters such as temperature, current density, exchange current density for anode and cathode, and the thickness of the electrolyte on the cell potential is investigated. Moreover, the overall performance of the electrolyzer is investigated through energy and exergy analysis. This study is demonstrated the electrochemical characteristics that are attached to the thermodynamic analysis of the electrolyzer. The current density-cell voltage (i-V) characteristic curve is a unique feature of its design which is generated at different operating parameters. Further, this curve for different losses such as ohmic and activation overpotentials are generated. The results show that the activation overpotential dominates the total voltage loss; the electrodes of the electrolyzer are more active at higher working temperatures leading to a lower activation overpotential; the cell potential decreases considerably with increasing i0 and increases significantly with an increase in electrolyte thickness due to higher ohmic overpotential; the electrolyzer works in an exothermic mode since the heat generation exceeds the thermal energy requirement because of the overpotentials. The experimental data existing in the literature are also compared with the current density-cell voltage (i-V) curve in the study that showed high similarity. The energy and exergy efficiency based on the H2 yield governed by the electrical energy input alone is almost the same (55~56%) since electricity has 100% exergy.
中文翻译:

碱性电解槽 (AE) 在不同操作参数下的热力学和电化学评估
在这项研究中,碱性电解槽在各种操作参数下进行了热力学和电化学分析,以获得最佳的氢气生产。研究了温度、电流密度、阳极和阴极的交换电流密度以及电解质厚度等不同操作参数对电池电位的影响。此外,通过能量和火用分析研究了电解槽的整体性能。本研究展示了与电解槽热力学分析相关的电化学特性。电流密度-电池电压 (iV) 特性曲线是其设计的一个独特特征,它是在不同的工作参数下产生的。此外,生成了针对不同损耗(例如欧姆和激活过电势)的这条曲线。结果表明,激活过电位在总电压损失中占主导地位;电解槽的电极在更高的工作温度下更活跃,导致更低的活化过电位;细胞电位随着 i 的增加而显着降低0并且由于较高的欧姆过电位而随着电解质厚度的增加而显着增加;电解槽以放热模式工作,因为过电位产生的热量超过了热能需求。文献中存在的实验数据也与研究中显示出高度相似性的电流密度-电池电压(iV)曲线进行了比较。基于仅由电能输入决定的 H 2产率的能量和火用效率几乎相同(55~56%),因为电具有 100% 的火用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号