当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
S-(Trifluoromethyl)Benzothioate (TFBT): A KF-Based Reagent for Nucleophilic Trifluoromethylthiolation
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2022-01-18 , DOI: 10.1002/chem.202104395 Depei Meng 1 , Yichong Lyu 1 , Chuanfa Ni 1 , Min Zhou 1 , Yang Li 2 , Jinbo Hu 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2022-01-18 , DOI: 10.1002/chem.202104395 Depei Meng 1 , Yichong Lyu 1 , Chuanfa Ni 1 , Min Zhou 1 , Yang Li 2 , Jinbo Hu 1
Affiliation
|
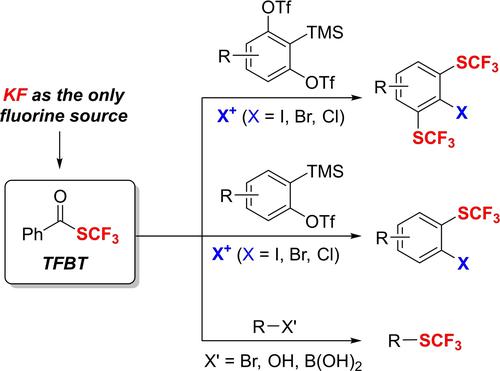
No need for HF: S-(Trifluoromethyl)benzothioate (TFBT), which is easily synthesized by using KF as the only fluorine source, has been developed as an inexpensive, bench-stable and user-friendly trifluoromethylthiolation reagent. TFBT reagent can readily release SCF3− with various counterions. The synthetic application of TFBT is demonstrated by trifluoromethylthiolation-halogenation of arynes, bis(trifluoromethylthiolation)-halogenation of 1,2-benzdiynes, nucleophilic substitution of alkyl halides, deoxytrifluoromethylthiolation of alcohols, and cross-coupling with aryl and vinyl boronic acids.
中文翻译:

S-(Trifluoromethyl)Benzothioate (TFBT):一种基于 KF 的亲核三氟甲基硫醇化试剂
无需 HF:S- (三氟甲基)苯硫代苯甲酸酯 (TFBT) 可通过使用 KF 作为唯一的氟源轻松合成,已被开发为廉价、稳定且用户友好的三氟甲基硫醇化试剂。TFBT 试剂可以很容易地释放 SCF 3 -与各种反离子。TFBT 的合成应用通过芳烃的三氟甲基硫醇化-卤化、1,2-苯二炔的双(三氟甲基硫醇化)-卤化、卤代烷的亲核取代、醇的脱氧三氟甲基硫醇化以及与芳基和乙烯基硼酸的交叉偶联来证明。
更新日期:2022-01-18
中文翻译:

S-(Trifluoromethyl)Benzothioate (TFBT):一种基于 KF 的亲核三氟甲基硫醇化试剂
无需 HF:S- (三氟甲基)苯硫代苯甲酸酯 (TFBT) 可通过使用 KF 作为唯一的氟源轻松合成,已被开发为廉价、稳定且用户友好的三氟甲基硫醇化试剂。TFBT 试剂可以很容易地释放 SCF 3 -与各种反离子。TFBT 的合成应用通过芳烃的三氟甲基硫醇化-卤化、1,2-苯二炔的双(三氟甲基硫醇化)-卤化、卤代烷的亲核取代、醇的脱氧三氟甲基硫醇化以及与芳基和乙烯基硼酸的交叉偶联来证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号