当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relative configuration of Cys-Pro ester peptides in thioester formation
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2022-01-18 , DOI: 10.1002/psc.3406 Toru Kawakami 1 , Eri Sasakura 1 , Yohei Miyanoiri 1 , Hironobu Hojo 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2022-01-18 , DOI: 10.1002/psc.3406 Toru Kawakami 1 , Eri Sasakura 1 , Yohei Miyanoiri 1 , Hironobu Hojo 1
Affiliation
|
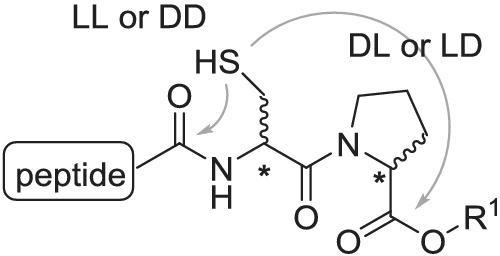
A peptide containing a cysteinyl prolyl ester (CPE) moiety at the C-terminus (CPE peptide) was transformed into a diketopiperazine (DKP) thioester via an intramolecular N–S acyl shift reaction and was then used for peptide ligation. The difference in reactivity between the CPE peptide stereoisomers was examined. In reactions of the CPE peptides that contained L-Cys-L-Pro or D-Cys-D-Pro, the desired DKP thioester was formed at the preceding amino acid residue. On the other hand, in reactions of the CPE peptides that contained D-Cys-L-Pro or L-Cys-D-Pro, a thiolactone was formed at the C-terminal prolyl ester, and the ligation occurred at the C-terminal Pro residue. Using this reaction, it was possible to efficiently prepare a cyclic peptide.
中文翻译:

Cys-Pro酯肽在硫酯形成中的相对构型
通过分子内 N-S 酰基转移反应,将 C 端含有半胱氨酰脯氨酰酯 (CPE) 部分的肽(CPE 肽)转化为二酮哌嗪 (DKP) 硫酯,然后用于肽连接。检查了 CPE 肽立体异构体之间的反应性差异。在含有 L-Cys-L-Pro 或 D-Cys-D-Pro 的 CPE 肽的反应中,所需的 DKP 硫酯在前面的氨基酸残基处形成。另一方面,在含有 D-Cys-L-Pro 或 L-Cys-D-Pro 的 CPE 肽的反应中,在 C-末端脯氨酰酯处形成硫代内酯,并在 C-末端发生连接。临残。使用该反应,可以有效地制备环状肽。
更新日期:2022-01-18
中文翻译:

Cys-Pro酯肽在硫酯形成中的相对构型
通过分子内 N-S 酰基转移反应,将 C 端含有半胱氨酰脯氨酰酯 (CPE) 部分的肽(CPE 肽)转化为二酮哌嗪 (DKP) 硫酯,然后用于肽连接。检查了 CPE 肽立体异构体之间的反应性差异。在含有 L-Cys-L-Pro 或 D-Cys-D-Pro 的 CPE 肽的反应中,所需的 DKP 硫酯在前面的氨基酸残基处形成。另一方面,在含有 D-Cys-L-Pro 或 L-Cys-D-Pro 的 CPE 肽的反应中,在 C-末端脯氨酰酯处形成硫代内酯,并在 C-末端发生连接。临残。使用该反应,可以有效地制备环状肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号