当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibrium and the Thermodynamic Correlation of Felodipine Form I in Pure and Mixed Solvents
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-18 , DOI: 10.1021/acs.jced.1c00566 Shasha Li 1, 2 , Xiaqing Nie 1, 2 , Chao Liu 1, 2 , Danhong Zhou 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-18 , DOI: 10.1021/acs.jced.1c00566 Shasha Li 1, 2 , Xiaqing Nie 1, 2 , Chao Liu 1, 2 , Danhong Zhou 1, 2
Affiliation
|
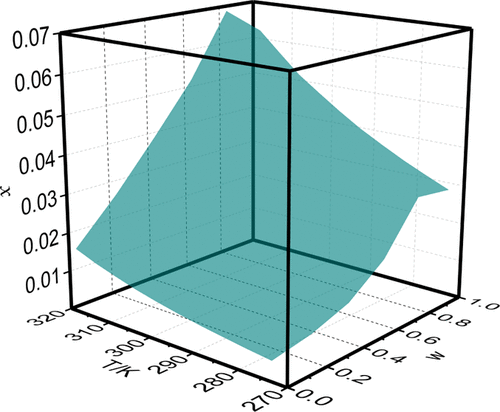
Because felodipine form I is used in marketed products, a co-solvent (ethyl acetate) is applied to enhance the solubility of poorly soluble form I in the present work. The solid–liquid equilibrium phase diagrams in pure and mixed solvents were established, and the effects of the solvent properties and intermolecular interaction on the solubility of felodipine were also studied. In pure solvent, the largest and smallest solubility data were obtained in ethyl acetate and toluene, respectively. The solubility increased to the maximum value at w = 0.8 with a mass fraction of ethyl acetate in (isopropanol + ethyl acetate) mixtures and then decreased with the increasing composition of the co-solvent. The modified Apelblat equation and the Jouyban–Acree model were applied to evaluate the solubility profile. All of the values of RAD were no more than 5%. More importantly, preferential solvation parameters (δx1,3) of felodipine in (isopropanol + ethyl acetate) mixtures were calculated using the inverse Kirkwood–Buff integrals method. The results indicated that the values of δx1,3 were all negative in ethyl acetate-rich and isopropanol-rich regions but positive in the composition of 0.45 < x1 < 0.80. Felodipine is preferentially solvated by isopropanol in ethyl acetate-rich and isopropanol-rich regions. In the composition of 0.45 < x1 < 0.80, felodipine is preferentially solvated by ethyl acetate.
中文翻译:

非洛地平 I 型在纯溶剂和混合溶剂中的固液相平衡和热力学相关性
由于非洛地平 I 型用于市售产品,因此在目前的工作中使用助溶剂(乙酸乙酯)来提高难溶性 I 型的溶解度。建立了纯溶剂和混合溶剂中的固液平衡相图,研究了溶剂性质和分子间相互作用对非洛地平溶解度的影响。在纯溶剂中,最大和最小的溶解度数据分别在乙酸乙酯和甲苯中获得。溶解度在w处增加到最大值= 0.8,乙酸乙酯在(异丙醇+乙酸乙酯)混合物中的质量分数,然后随着助溶剂组成的增加而降低。应用改进的 Apelblat 方程和 Jouyban-Acree 模型来评估溶解度曲线。RAD的所有值都不超过5%。更重要的是,使用逆柯克伍德-布夫积分法计算非洛地平在(异丙醇 + 乙酸乙酯)混合物中的优先溶剂化参数 (δ x 1,3 )。结果表明 δ x 1,3在乙酸乙酯富集区和异丙醇富集区均为负值,而在 0.45 < x 1的成分中为正值< 0.80。在富含乙酸乙酯和富含异丙醇的区域,非洛地平优先被异丙醇溶剂化。在 0.45 < x 1 < 0.80 的组成中,非洛地平优先被乙酸乙酯溶剂化。
更新日期:2022-02-10
中文翻译:

非洛地平 I 型在纯溶剂和混合溶剂中的固液相平衡和热力学相关性
由于非洛地平 I 型用于市售产品,因此在目前的工作中使用助溶剂(乙酸乙酯)来提高难溶性 I 型的溶解度。建立了纯溶剂和混合溶剂中的固液平衡相图,研究了溶剂性质和分子间相互作用对非洛地平溶解度的影响。在纯溶剂中,最大和最小的溶解度数据分别在乙酸乙酯和甲苯中获得。溶解度在w处增加到最大值= 0.8,乙酸乙酯在(异丙醇+乙酸乙酯)混合物中的质量分数,然后随着助溶剂组成的增加而降低。应用改进的 Apelblat 方程和 Jouyban-Acree 模型来评估溶解度曲线。RAD的所有值都不超过5%。更重要的是,使用逆柯克伍德-布夫积分法计算非洛地平在(异丙醇 + 乙酸乙酯)混合物中的优先溶剂化参数 (δ x 1,3 )。结果表明 δ x 1,3在乙酸乙酯富集区和异丙醇富集区均为负值,而在 0.45 < x 1的成分中为正值< 0.80。在富含乙酸乙酯和富含异丙醇的区域,非洛地平优先被异丙醇溶剂化。在 0.45 < x 1 < 0.80 的组成中,非洛地平优先被乙酸乙酯溶剂化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号