Applied Surface Science ( IF 6.3 ) Pub Date : 2022-01-17 , DOI: 10.1016/j.apsusc.2022.152489 Chengwen Wang 1 , Lei Sun 1 , Qingqing Wang 1 , Yanxiu Wang 1 , Yang Cao 1 , Xin Wang 1 , Ping Chen 1 , Wei Sun 1
|
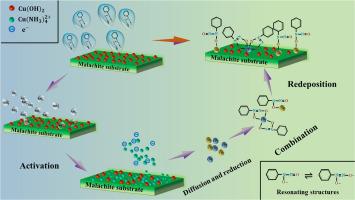
The adsorption mechanism between malachite and a specific organic ligand of the ammonium salt of N-Nitroso-N-phenylhydroxyamine (NBH) with the NH4+ group and nitrosamine polar group has been studied. The tapping mode atomic force microscopy (AFM) imaging illustrated that irregular protrusions were covered on malachite surface at the nanoscale when malachite was treated in NBH solutions under alkaline conditions at pH 9.5. It indicated that the NBH displayed an excellent affinity to malachite, and the adsorption density was correlated with the concentration of NBH. Based on copper ammine theory and the results of Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS), the intermediate transition process of the interaction between malachite and NBH can be summarized as activation, diffusion and reduction, combination, and redeposition. The copper ions removed from the ammonia-activated malachite surface combined with the NBH resonating structures to form the bidentate and monodentate complexes. Eventually, those microscopic analyses are confirmed by the macroscopic flotation test. The flotation recovery illustrated the formation of ligand–Cu complex strengthened the hydrophobicity of malachite in a three-phase system. Therefore, it is feasible using NBH as collector to selectively separate malachite from gangue minerals in flotation experiments.
中文翻译:

N-亚硝基-N-苯基羟胺铵盐对孔雀石矿物的吸附机理及浮选行为
孔雀石与N-亚硝基-N-苯基羟基胺(NBH)铵盐的特定有机配体与NH 4 +的吸附机理基团和亚硝胺极性基团进行了研究。轻敲模式原子力显微镜 (AFM) 成像表明,当孔雀石在 NBH 溶液中在 pH 9.5 的碱性条件下处理时,孔雀石表面覆盖了纳米级的不规则突起。说明 NBH 对孔雀石具有良好的亲和力,吸附密度与 NBH 浓度相关。基于铜氨理论和傅里叶变换红外光谱(FTIR)和X射线光电子能谱(XPS)的结果,孔雀石与NBH相互作用的中间过渡过程可以概括为活化、扩散和还原、结合、和再沉积。从氨活化的孔雀石表面去除的铜离子与 NBH 共振结构结合形成双齿和单齿配合物。最终,这些微观分析通过宏观浮选试验得到证实。浮选回收表明配体-铜络合物的形成增强了孔雀石在三相体系中的疏水性。因此,在浮选实验中,使用 NBH 作为捕收剂将孔雀石与脉石矿物选择性分离是可行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号