Applied Surface Science ( IF 6.3 ) Pub Date : 2022-01-10 , DOI: 10.1016/j.apsusc.2022.152474 Yamei Zeng 1 , Xu Zhang 1 , Changzhi Ai 1 , Caizhuang Wang 2 , Yipu Liu 1 , Shiwei Lin 1
|
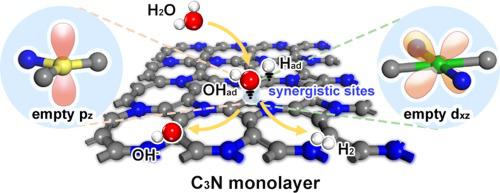
Alkaline water electrolyzer (AWE) is one of the promising technologies for hydrogen production at the industrial level. However, energetic inefficiency and low current density impede the development of AWE. Compared with acidic conditions, the Volmer step in alkaline hydrogen evolution reaction (HER) involves extra water dissociation, whose barrier is one of the most vital reasons for the sluggish kinetics of alkaline HER. Herein, choosing C3N monolayer as an ideal theoretical model, we design several empty orbitals through intentional metal doping, and further construct synergistic sites on the C3N monolayer to accelerate both water dissociation and hydrogen adsorption for alkaline HER. The as-designed Be-doped and Cr-doped C3N monolayers exhibit rather low theoretical overpotential of 0.476 eV and 0.216 eV for alkaline HER, respectively, which are even lower than Pt (1 1 1) surface. Moreover, by comparing the water dissociation behaviors on metal-doped C3N monolayer, we find that the empty orbitals with suitable orientation and energy level are useful for promoting the water dissociation process, indicating that we can use orbital engineering strategy to regulate the adsorption strength between adsorbate and surface site. Consequently, it is reasonable to suggest that our orbital engineering strategy would significantly benefit the design of highly efficient alkaline HER electrocatalysts.
中文翻译:

C3N单层轨道工程设计高效协同位点电催化剂以促进碱性析氢
碱性水电解槽(AWE)是工业级制氢的有前途的技术之一。然而,能量低效率和低电流密度阻碍了AWE的发展。与酸性条件相比,碱性析氢反应 (HER) 中的 Volmer 步骤涉及额外的水解离,其屏障是碱性 HER 动力学缓慢的最重要原因之一。在此,我们选择C 3 N 单层作为理想的理论模型,通过有意的金属掺杂设计了几个空轨道,并进一步在C 3 N 单层上构建协同位点,以加速碱性HER 的水解离和氢吸附。所设计的 Be 掺杂和 Cr 掺杂 C 3N单层对碱性HER表现出相当低的理论过电势,分别为0.476 eV和0.216 eV,甚至低于Pt(1 1 1)表面。此外,通过比较金属掺杂C 3 N 单分子层上的水解离行为,我们发现具有合适取向和能级的空轨道有助于促进水解离过程,表明我们可以使用轨道工程策略来调节吸附吸附质和表面位点之间的强度。因此,有理由认为我们的轨道工程策略将显着有利于高效碱性 HER 电催化剂的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号