Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-01-13 , DOI: 10.1016/j.cej.2022.134674 Fengzhen Zhang 1 , Qiaoping Kong 2 , Huihuang Chen 1 , Xu Zhao 3 , Bo Yang 1 , Sergei Preis 4
|
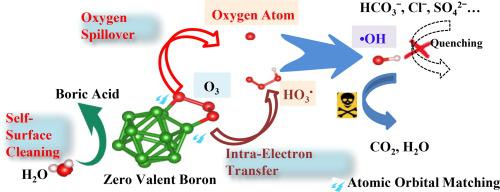
Catalytic ozonation process (COP) for degrading pollutants is limited by inefficient recovery of reactive sites on catalyst surface and scavenging of hydroxyl radical (•OH) by inorganic anions. Herein, we employed zero valent boron (ZVB) as a catalyst overcoming the abovementioned shortcomings. The established ZVB/O3 had outstanding capability to generate •OH for near-complete decontamination in seconds with normalized kinetic coefficients far surpassing most reported works. The spontaneous dissolution of B2O3 passive layer and ZVB-mediated valence circulation of adsorbed SO42− anion ensured sustainable reactive sites recovery and anti-scavenging of •OH by SO42− anion, respectively. The other common inorganic anions (Cl−, HCO3− and H2PO4−) showed negligible scavenging effects on •OH-mediated reactions. Density functional theory calculation and experimental results revealed that auto-matching the empty 2p orbital of boron atom with lone pair electrons of oxygen atom in ozone played crucial roles in forming coordination bonds, resulting in strong ozone chemical adsorption by boron atom on ZVB surface. The adsorbed ozone was transformed into atomic oxygen and then •OH via a novel oxygen spillover mechanism. Moreover, the B-B bond on ZVB surface was verified to be another active site for ozone activation into •OH by intra-electron transfer mechanism which was promoted by the in-situ formed coordination bonds between ozone and ZVB. This work establishes next-generation COP by eliminating surface- and bulk-phase reaction limitations.
中文翻译:

用于超快速降解有机污染物的零价硼活化臭氧化:原子轨道匹配、氧溢出和电子内转移
用于降解污染物的催化臭氧化工艺 (COP) 受到催化剂表面反应位点回收效率低下和无机阴离子清除羟基自由基 (•OH) 的限制。在此,我们采用零价硼(ZVB)作为催化剂,克服了上述缺点。已建立的 ZVB/O 3具有出色的生成 •OH 的能力,可在几秒钟内完成近乎完全的去污,归一化动力学系数远远超过大多数报道的工作。B 2 O 3钝化层的自发溶解和吸附的 SO 4 2-阴离子的 ZVB 介导的价循环确保了可持续的反应位点恢复和 SO 4 2-对 •OH 的抗清除阴离子,分别。其他常见的无机阴离子(Cl -、HCO 3 -和 H 2 PO 4 -) 对•OH 介导的反应显示出可忽略不计的清除作用。密度泛函理论计算和实验结果表明,硼原子的空2p轨道与臭氧中氧原子的孤对电子自匹配对形成配位键起关键作用,导致硼原子在ZVB表面产生强烈的臭氧化学吸附。吸附的臭氧通过一种新的氧溢出机制转化为原子氧,然后转化为•OH。此外,通过臭氧和ZVB之间原位形成的配位键促进的电子内转移机制,证实ZVB表面的BB键是臭氧活化成•OH的另一个活性位点。这项工作通过消除表面和体相反应限制建立了下一代 COP。









































 京公网安备 11010802027423号
京公网安备 11010802027423号