当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable-Phase Diagram of the Quaternary Water–Salt System K+, Rb+, Cs+//SO42––H2O at T = 323.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.jced.1c00643 Ying Zeng 1, 2 , Ke Wang 1 , Hanlin Tong 1 , Xinxin Lv 1 , Yuhua He 1 , Xudong Yu 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.jced.1c00643 Ying Zeng 1, 2 , Ke Wang 1 , Hanlin Tong 1 , Xinxin Lv 1 , Yuhua He 1 , Xudong Yu 1, 2
Affiliation
|
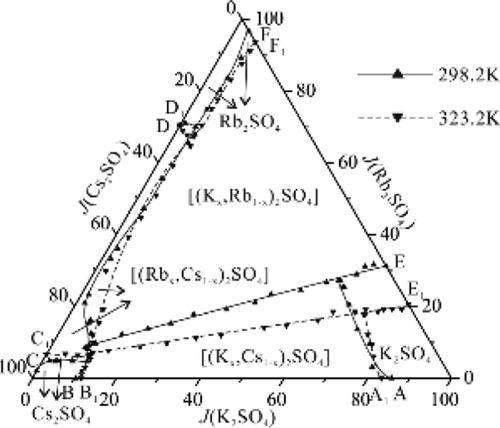
The stable-phase equilibria of the aqueous quaternary system K+, Rb+, Cs+//SO42––H2O was investigated at T = 323.2 K using the isothermal dissolution equilibrium method. The solubility, density, and refractive index of equilibrium solutions were determined using the chemical/instrument analysis method, the specific gravity bottle method, and the WYA Abbe refractometer, respectively. The solid phases were identified by using X-ray diffraction (XRD) and scanning electron microscopy (SEM). The results reveal that besides three single salts (K2SO4, Rb2SO4, and Cs2SO4), three complete solid solutions are formed in this system at 323.2 K, namely, [(Kx, Rb1–x)2SO4], [(Kx, Cs1–x)2SO4], and [(Rbx, Cs1–x)2SO4], increasing the difficulty of separation potassium from the solution composed of rubidium, cesium, and sulfate. The stable-phase diagram of the quaternary system K+, Rb+, Cs+//SO42––H2O at T = 323.2 K consists of four invariant points, nine univariant curves, and six crystallization regions. The sequence of the size of the salt crystal region is [(Kx, Rb1–x)2SO4] > [(Kx, Cs1–x)2SO4] > K2SO4 > [(Rbx, Cs1–x)2SO4] > Rb2SO4 > Cs2SO4. The phase diagrams at different temperatures (T = 298.2 K and T = 323.2 K) show that temperature is an important factor affecting salt crystallization, especially for the solid solution. As the temperature increases, the crystallization region of [(Kx, Cs1–x)2SO4] conspicuously decreases, whereas the crystallization regions of [(Kx, Rb1–x)2SO4] and [(Rbx, Cs1–x)2SO4] increase slightly.
中文翻译:

T = 323.2 K 时第四纪水-盐系统 K+, Rb+, Cs+//SO42––H2O 的稳定相图
采用等温溶解平衡法在T = 323.2 K 时研究了水性四元体系 K +、Rb +、Cs + //SO 4 2– -H 2 O的稳定相平衡。平衡溶液的溶解度、密度和折光率分别采用化学/仪器分析法、比重瓶法和WYA阿贝折光仪测定。通过使用X射线衍射(XRD)和扫描电子显微镜(SEM)鉴定固相。结果表明,除了三种单盐(K 2 SO 4、Rb 2 SO 4和 Cs2 SO 4 ),该体系在323.2 K时形成三种完全固溶体,即[(K x , Rb 1– x ) 2 SO 4 ], [(K x , Cs 1– x ) 2 SO 4 ],和[(Rb x , Cs 1– x ) 2 SO 4 ],增加了从铷、铯和硫酸盐组成的溶液中分离钾的难度。四元系K + , Rb + , Cs + //SO 4 2– –H的稳定相图T = 323.2 K 的2 O由四个不变点、九个单变量曲线和六个结晶区域组成。盐晶区大小顺序为[(K x , Rb 1– x ) 2 SO 4 ] > [(K x , Cs 1– x ) 2 SO 4 ] > K 2 SO 4 > [(Rb x , Cs 1– x ) 2 SO 4 ] > Rb 2 SO 4 > Cs 2 SO 4。不同温度下的相图(T = 298.2 K 和T = 323.2 K)表明温度是影响盐结晶的重要因素,特别是对于固溶体。随着温度的升高,[(K x , Cs 1– x ) 2 SO 4 ]的结晶区明显减小,而[(K x , Rb 1– x ) 2 SO 4 ]和[(Rb x , Cs 1– x ) 2 SO 4 ] 略有增加。
更新日期:2022-02-10
中文翻译:

T = 323.2 K 时第四纪水-盐系统 K+, Rb+, Cs+//SO42––H2O 的稳定相图
采用等温溶解平衡法在T = 323.2 K 时研究了水性四元体系 K +、Rb +、Cs + //SO 4 2– -H 2 O的稳定相平衡。平衡溶液的溶解度、密度和折光率分别采用化学/仪器分析法、比重瓶法和WYA阿贝折光仪测定。通过使用X射线衍射(XRD)和扫描电子显微镜(SEM)鉴定固相。结果表明,除了三种单盐(K 2 SO 4、Rb 2 SO 4和 Cs2 SO 4 ),该体系在323.2 K时形成三种完全固溶体,即[(K x , Rb 1– x ) 2 SO 4 ], [(K x , Cs 1– x ) 2 SO 4 ],和[(Rb x , Cs 1– x ) 2 SO 4 ],增加了从铷、铯和硫酸盐组成的溶液中分离钾的难度。四元系K + , Rb + , Cs + //SO 4 2– –H的稳定相图T = 323.2 K 的2 O由四个不变点、九个单变量曲线和六个结晶区域组成。盐晶区大小顺序为[(K x , Rb 1– x ) 2 SO 4 ] > [(K x , Cs 1– x ) 2 SO 4 ] > K 2 SO 4 > [(Rb x , Cs 1– x ) 2 SO 4 ] > Rb 2 SO 4 > Cs 2 SO 4。不同温度下的相图(T = 298.2 K 和T = 323.2 K)表明温度是影响盐结晶的重要因素,特别是对于固溶体。随着温度的升高,[(K x , Cs 1– x ) 2 SO 4 ]的结晶区明显减小,而[(K x , Rb 1– x ) 2 SO 4 ]和[(Rb x , Cs 1– x ) 2 SO 4 ] 略有增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号