当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism and Solubility Performance Evaluation for Separation of Benzothiophene and Model Diesel Compounds through Deep Eutectic Solvents as Extractants
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.iecr.1c04083 Nikhil Kumar 1 , Tamal Banerjee 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.iecr.1c04083 Nikhil Kumar 1 , Tamal Banerjee 1
Affiliation
|
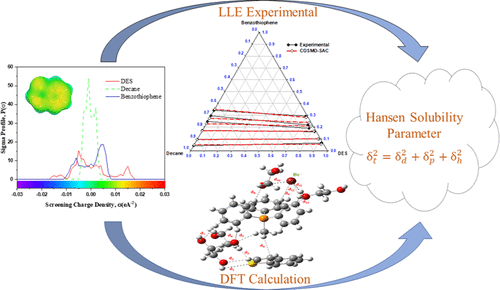
During combustion, sulfur compounds in fuels are oxidized to SOx, poisoning automatic catalytic converters and posing severe environmental risks (e.g., acid rain). As an outcome, effective liquid fuel desulphurization is a vital step in reducing SOx emissions and their inherent ecological impacts. The extraction of ultralow benzothiophene from n-decane as representative model diesel fuel was analyzed using phosphonium-based deep eutectic solvents (DESs) at 308.15 K and 1 bar pressure. Liquid–liquid equilibrium (LLE) studies were conducted on a ternary system of DES–benzothiophene–n-decane for all six feed point concentrations. 1H NMR analysis was used to determine the molar composition of each raffinate and extract phase. The LLE experimental findings were examined using a conductor-like screening model for segment activity coefficient (COSMO-SAC) based on quantum calculations. The average root-mean-square deviation (RMSD) of the studied LLE ternary system was 1.07%, which was suitable considering the prior nature of the approach. The σ-profile calculations using the COSMO-SAC model and Hansen solubility parameters (HSPs) using different predicted models were used to provide insights into the molecular mechanism of benzothiophene extraction efficacy using DES. We observed higher extraction of benzothiophene due to the higher proportion of hydrogen-bonding and dispersion-type interactions. Charge transfer analysis using the density functional theory (DFT) calculation of the DES cluster suggested that bromide (Br–) ions can act as a charge transfer bridge between the methyltriphenylphosphonium (MTP) cation and the hydrogen bond donor. The MTP cation and ethylene glycol also aided in the extraction of benzothiophene by dislocating charge from the aromatic sulfur compound. The bromide (Br–) ion is essentially noninteractive with benzothiophene due to retention of the conventional hydrogen bond network existing within the initial components of DES, mainly hydrogen bond donors and hydrogen bond acceptors.
中文翻译:

深共晶溶剂作为萃取剂分离苯并噻吩和模型柴油化合物的分子机理和溶解性能评价
在燃烧过程中,燃料中的硫化合物被氧化成 SO x,使自动催化转化器中毒并造成严重的环境风险(例如酸雨)。因此,有效的液体燃料脱硫是减少 SO x排放及其内在生态影响的关键步骤。在 308.15 K 和 1 bar 压力下,使用基于鏻的低共熔溶剂 (DES) 分析了从正癸烷中提取超低苯并噻吩作为代表性模型柴油燃料的情况。对所有六个进料点浓度的 DES-苯并噻吩-正癸烷三元系统进行了液-液平衡 (LLE) 研究。1H NMR分析用于确定每个萃余液和萃取相的摩尔组成。使用基于量子计算的节段活动系数 (COSMO-SAC) 的类导体筛选模型检查 LLE 实验结果。所研究的 LLE 三元系统的平均均方根偏差 (RMSD) 为 1.07%,考虑到该方法的先验性质,这是合适的。使用 COSMO-SAC 模型和使用不同预测模型的 Hansen 溶解度参数 (HSP) 的 σ 曲线计算用于深入了解使用 DES 的苯并噻吩提取功效的分子机制。由于氢键和分散型相互作用的比例更高,我们观察到苯并噻吩的提取率更高。– ) 离子可以充当甲基三苯基鏻 (MTP) 阳离子和氢键供体之间的电荷转移桥。MTP 阳离子和乙二醇也有助于通过从芳族硫化合物中置换电荷来萃取苯并噻吩。由于 DES 初始组分(主要是氢键供体和氢键受体)中存在的常规氢键网络的保留,溴化物 (Br – ) 离子基本上与苯并噻吩不相互作用。
更新日期:2022-01-26
中文翻译:

深共晶溶剂作为萃取剂分离苯并噻吩和模型柴油化合物的分子机理和溶解性能评价
在燃烧过程中,燃料中的硫化合物被氧化成 SO x,使自动催化转化器中毒并造成严重的环境风险(例如酸雨)。因此,有效的液体燃料脱硫是减少 SO x排放及其内在生态影响的关键步骤。在 308.15 K 和 1 bar 压力下,使用基于鏻的低共熔溶剂 (DES) 分析了从正癸烷中提取超低苯并噻吩作为代表性模型柴油燃料的情况。对所有六个进料点浓度的 DES-苯并噻吩-正癸烷三元系统进行了液-液平衡 (LLE) 研究。1H NMR分析用于确定每个萃余液和萃取相的摩尔组成。使用基于量子计算的节段活动系数 (COSMO-SAC) 的类导体筛选模型检查 LLE 实验结果。所研究的 LLE 三元系统的平均均方根偏差 (RMSD) 为 1.07%,考虑到该方法的先验性质,这是合适的。使用 COSMO-SAC 模型和使用不同预测模型的 Hansen 溶解度参数 (HSP) 的 σ 曲线计算用于深入了解使用 DES 的苯并噻吩提取功效的分子机制。由于氢键和分散型相互作用的比例更高,我们观察到苯并噻吩的提取率更高。– ) 离子可以充当甲基三苯基鏻 (MTP) 阳离子和氢键供体之间的电荷转移桥。MTP 阳离子和乙二醇也有助于通过从芳族硫化合物中置换电荷来萃取苯并噻吩。由于 DES 初始组分(主要是氢键供体和氢键受体)中存在的常规氢键网络的保留,溴化物 (Br – ) 离子基本上与苯并噻吩不相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号