当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondria-Targeting Phototheranostics by Aggregation-Induced NIR-II Emission Luminogens: Modulating Intramolecular Motion by Electron Acceptor Engineering for Multi-Modal Synergistic Therapy
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-01-10 , DOI: 10.1002/adfm.202110526 Tianfu Zhang 1 , Jianyu Zhang 1 , Fu‐Bing Wang 2 , Hui Cao 3, 4 , Daoming Zhu 5 , Xiaoyu Chen 6 , Changhuo Xu 1 , Xueqin Yang 1 , Wenbin Huang 7 , Zhaoyu Wang 1 , Jiefei Wang 6 , Zikai He 7 , Zheng Zheng 3, 4 , Jacky Wing Yip Lam 1 , Ben Zhong Tang 1, 8
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2022-01-10 , DOI: 10.1002/adfm.202110526 Tianfu Zhang 1 , Jianyu Zhang 1 , Fu‐Bing Wang 2 , Hui Cao 3, 4 , Daoming Zhu 5 , Xiaoyu Chen 6 , Changhuo Xu 1 , Xueqin Yang 1 , Wenbin Huang 7 , Zhaoyu Wang 1 , Jiefei Wang 6 , Zikai He 7 , Zheng Zheng 3, 4 , Jacky Wing Yip Lam 1 , Ben Zhong Tang 1, 8
Affiliation
|
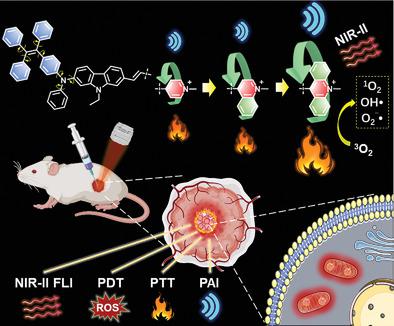
Phototheranostic agents have thrived as promising tools for cancer theranostics because of the integration of sensitive in situ fluorescence imaging and effective multi-model synergistic therapy. However, how to manipulate the intangible photon energy transfer to balance the competitive radiative and nonradiative processes is still challenging. Although numerous phototheranostic molecules are reported, their complicated molecular design and tedious synthesis often stumble further their development. Herein, three simple molecules with electron donating−accepting structures are developed. The electron acceptor engineering on molecules by introducing acridinium unit gives rise to TPEDCAc with aggregation-induced second near-infrared emission (AIE NIR-II), high reactive oxygen species generation capability, and excellent photothermal conversion efficiency (44.8%) due to the drastic intramolecular motion of large acridinium rotor and balanced AIE effect. Experimental analysis and calculation on the controlled molecules suggested that large torsional angle and the strong electron-withdrawing ability of the acridinium unit are keys for NIR-II emission and balanced photodynamic/photothermal conversion. Impressively, the positively charged TPEDCAc shows mitochondria-targeting capability and high performance in in vivo multi-modal cancer theranostics under NIR laser irradiation. Hence, this work not only provides a single NIR-II AIE-based multi-modal cancer theranostic system but inspires new insights into future development of new theranostic platforms.
中文翻译:

通过聚集诱导的 NIR-II 发射发光剂靶向线粒体的光疗:通过电子受体工程调节分子内运动用于多模式协同治疗
由于集成了灵敏的原位荧光成像和有效的多模型协同治疗,光治疗剂已成为癌症治疗学的有前途的工具。然而,如何操纵无形光子能量转移以平衡竞争性辐射和非辐射过程仍然具有挑战性。尽管报道了许多光疗分子,但它们复杂的分子设计和繁琐的合成往往阻碍了它们的进一步发展。在此,开发了三种具有给电子-接受结构的简单分子。通过引入吖啶单元对分子进行电子受体工程,产生具有聚集诱导的第二近红外发射 (AIE NIR-II)、高活性氧生成能力的 TPEDCAc,由于大吖啶转子的剧烈分子内运动和平衡的AIE效应,具有出色的光热转换效率(44.8%)。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。
更新日期:2022-01-10
中文翻译:

通过聚集诱导的 NIR-II 发射发光剂靶向线粒体的光疗:通过电子受体工程调节分子内运动用于多模式协同治疗
由于集成了灵敏的原位荧光成像和有效的多模型协同治疗,光治疗剂已成为癌症治疗学的有前途的工具。然而,如何操纵无形光子能量转移以平衡竞争性辐射和非辐射过程仍然具有挑战性。尽管报道了许多光疗分子,但它们复杂的分子设计和繁琐的合成往往阻碍了它们的进一步发展。在此,开发了三种具有给电子-接受结构的简单分子。通过引入吖啶单元对分子进行电子受体工程,产生具有聚集诱导的第二近红外发射 (AIE NIR-II)、高活性氧生成能力的 TPEDCAc,由于大吖啶转子的剧烈分子内运动和平衡的AIE效应,具有出色的光热转换效率(44.8%)。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。对受控分子的实验分析和计算表明,吖啶单元的大扭转角和强吸电子能力是NIR-II发射和平衡光动力/光热转换的关键。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。令人印象深刻的是,带正电荷的 TPEDCAc 在近红外激光照射下在体内多模式癌症治疗中显示出线粒体靶向能力和高性能。因此,这项工作不仅提供了一个单一的基于 NIR-II AIE 的多模式癌症治疗诊断系统,而且激发了对新治疗诊断平台未来发展的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号