当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nisoldipine in Several Aqueous and Non-Aqueous Cosolvent Solutions: Solubility Modeling and Preferential Solvation Research
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-13 , DOI: 10.1021/acs.jced.1c00729 Fang Zhang 1 , Fuwei Pan 1 , Hairong Wang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-01-13 , DOI: 10.1021/acs.jced.1c00729 Fang Zhang 1 , Fuwei Pan 1 , Hairong Wang 1
Affiliation
|
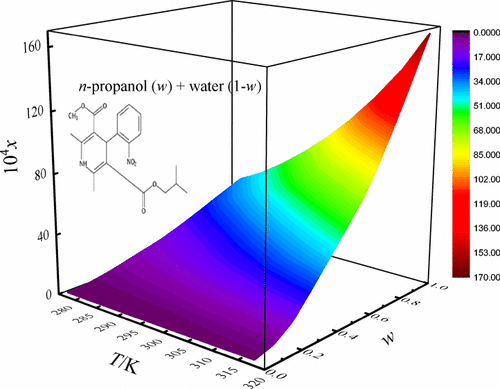
The study mainly reported the equilibrated solubilities of nisoldipine in aqueous solutions of n-propanol/acetonitrile + water and non-aqueous solutions of ethyl acetate + ethanol/isopropanol by dint of experimentation and model correlations. Solubility was experimented via the shake-flask method under 101.2 kPa from 278.15 to 318.15 K. The maximum of nisoldipine solubility (in mole fraction unit) was found in neat n-propanol/acetonitrile/ethyl acetate at 318.15 K, and the minimum was found in neat water/isopropanol/ethanol. The total and three-dimensional Hansen solubility parameters were employed herein to explain the solubility behavior of nisoldipine. Modeling solubility through mixture response surface, Jouyban–Acree, and Jouyban–Acree–van’t Hoff models resulted in relative average deviations of <10.3%. Quantitative research upon preferential solvation was carried out by applying the approach of inverse Kirkwood–Buff integrals for the four solution systems considered in the present study and two aqueous solutions of ethanol/isopropanol reported in the literature. The local fractions of n-propanol/acetonitrile/ethanol/isopropanol for the four aqueous solutions as well as ethyl acetate for non-aqueous solution of ethyl acetate + isopropanol were greater than that of bulk ones in n-propanol/acetonitrile/ethanol/isopropanol/ethyl acetate-rich and middle composition proportions, demonstrating that the solvation of nisoldipine was preferred by cosolvents (n-propanol/acetonitrile/ethanol/isopropanol/ethyl acetate). It is able to speculate that nisoldipine displays Lewis acid behavior in these regions with the cosolvent molecules in aqueous solution systems and with the ethyl acetate molecules or isopropanol in the ethyl acetate + isopropanol solution system.
中文翻译:

几种水性和非水性助溶剂溶液中的尼索地平:溶解度建模和优先溶剂化研究
该研究主要通过实验和模型相关性报告了尼索地平在正丙醇/乙腈+水的水溶液和乙酸乙酯+乙醇/异丙醇的非水溶液中的平衡溶解度。在 278.15 至 318.15 K 下,通过摇瓶法在 101.2 kPa 下对溶解度进行了实验。尼索地平溶解度的最大值(以摩尔分数为单位)在纯n-丙醇/乙腈/乙酸乙酯,318.15 K,在纯水/异丙醇/乙醇中发现最小值。本文采用总和三维汉森溶解度参数来解释尼索地平的溶解度行为。通过混合物响应面、Jouyban-Acree 和 Jouyban-Acree-van't Hoff 模型对溶解度进行建模,导致相对平均偏差 <10.3%。通过对本研究中考虑的四种溶液系统和文献中报道的两种乙醇/异丙醇水溶液应用逆 Kirkwood-Buff 积分的方法,对优先溶剂化进行了定量研究。n的局部分数4种水溶液的-丙醇/乙腈/乙醇/异丙醇以及乙酸乙酯+异丙醇的非水溶液的乙酸乙酯大于正丙醇/乙腈/乙醇/异丙醇/乙酸乙酯富集的本体溶液和中间的组成比例,表明尼索地平的溶剂化是优选的共溶剂(正丙醇/乙腈/乙醇/异丙醇/乙酸乙酯)。可以推测尼索地平在这些区域与水溶液体系中的助溶剂分子以及乙酸乙酯+异丙醇溶液体系中的乙酸乙酯分子或异丙醇表现出路易斯酸行为。
更新日期:2022-02-10
中文翻译:

几种水性和非水性助溶剂溶液中的尼索地平:溶解度建模和优先溶剂化研究
该研究主要通过实验和模型相关性报告了尼索地平在正丙醇/乙腈+水的水溶液和乙酸乙酯+乙醇/异丙醇的非水溶液中的平衡溶解度。在 278.15 至 318.15 K 下,通过摇瓶法在 101.2 kPa 下对溶解度进行了实验。尼索地平溶解度的最大值(以摩尔分数为单位)在纯n-丙醇/乙腈/乙酸乙酯,318.15 K,在纯水/异丙醇/乙醇中发现最小值。本文采用总和三维汉森溶解度参数来解释尼索地平的溶解度行为。通过混合物响应面、Jouyban-Acree 和 Jouyban-Acree-van't Hoff 模型对溶解度进行建模,导致相对平均偏差 <10.3%。通过对本研究中考虑的四种溶液系统和文献中报道的两种乙醇/异丙醇水溶液应用逆 Kirkwood-Buff 积分的方法,对优先溶剂化进行了定量研究。n的局部分数4种水溶液的-丙醇/乙腈/乙醇/异丙醇以及乙酸乙酯+异丙醇的非水溶液的乙酸乙酯大于正丙醇/乙腈/乙醇/异丙醇/乙酸乙酯富集的本体溶液和中间的组成比例,表明尼索地平的溶剂化是优选的共溶剂(正丙醇/乙腈/乙醇/异丙醇/乙酸乙酯)。可以推测尼索地平在这些区域与水溶液体系中的助溶剂分子以及乙酸乙酯+异丙醇溶液体系中的乙酸乙酯分子或异丙醇表现出路易斯酸行为。









































 京公网安备 11010802027423号
京公网安备 11010802027423号