Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2022-01-13 , DOI: 10.1016/j.jcou.2022.101888 Adrián Quindimil 1 , Jon A. Onrubia-Calvo 1 , Arantxa Davó-Quiñonero 2 , Alejandro Bermejo-López 1 , Esther Bailón-García 2 , Beñat Pereda-Ayo 1 , Dolores Lozano-Castelló 2 , José A. González-Marcos 1 , Agustín Bueno-López 2 , Juan R. González-Velasco 1
|
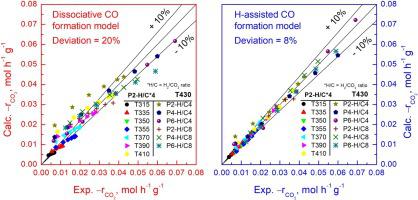
The mechanism and kinetic of CO2 methanation reaction of 9.5 % Ni/Al2O3 catalyst is analysed under a wide range of operating conditions. Once the catalyst activity is stabilized, the influence of temperature, total pressure and space velocity is studied for kinetic characterisation. A data set comprising of 153 experimental runs has been used to develop a kinetic model capable to accurately predict the reaction rate. Ni/Al2O3 catalyst shows an apparent activation energy of 80.1 kJ mol−1 in CO2 hydrogenation. Data obtained under differential mode adjust quite precisely to a power-law model with H2O inhibition, with a water adsorption constant of 3.1 atm−1 and apparent orders of 0.24 and 0.27 for H2 and CO2, respectively. Based on DRIFTS results, we propose for the first time the H-assisted CO formation route, which is compared with the more conventionally reported carbonyl route, and describe the corresponding reaction rate LHHW equation, resulting in notable improvement for mean deviation (D) of 7.0 % in our model related to that based on the carbonyl route (D = 20.1 %) usually suggested for catalysts with higher Ni loads around 20 %. The H-assisted CO formation route considers the formate species decomposition into carbonyls via H-assisted CO formation mechanism and further carbonyls hydrogenation into CHO as the rate determining step. Thus, the LHHW mechanism, in which carbonyls as well as formate species participate in CO2 methanation, is capable to reflect the kinetics of lowly-loaded Ni/Al2O 3 catalyst with high accuracy under relevant process conditions (315−430 °C, 1−6 bar, H2 to CO2 molar ratios between 1–16 and, different reagents and products partial pressures).
中文翻译:

低负载 Ni/Al2O3 催化剂上 CO2 甲烷化的内在动力学:机理、模型判别和参数估计
分析了9.5% Ni/Al 2 O 3催化剂在各种操作条件下的CO 2甲烷化反应机理和动力学。一旦催化剂活性稳定,就研究温度、总压力和空速的影响以进行动力学表征。包含 153 次实验运行的数据集已用于开发能够准确预测反应速率的动力学模型。Ni/Al 2 O 3催化剂在CO 2加氢中显示出80.1 kJ mol -1的表观活化能。在差分模式下获得的数据非常精确地调整为具有 H 2的幂律模型O抑制,水吸附常数为3.1 atm -1 ,H 2和CO 2的表观数量分别为0.24和0.27 。基于 DRIFTS 结果,我们首次提出了 H 辅助 CO 形成路线,该路线与更常规报道的羰基路线进行了比较,并描述了相应的反应速率 LHHW 方程,从而显着改善了平均偏差 ( D )我们模型中的 7.0 % 与基于羰基路线的模型相关(D= 20.1 %) 通常建议用于具有较高 Ni 负载量约 20 % 的催化剂。H 辅助 CO 形成路线将甲酸盐物质通过 H 辅助 CO 形成机制分解为羰基化合物,并将羰基化合物进一步氢化为 CHO 作为速率决定步骤。因此,羰基和甲酸盐参与CO 2甲烷化的LHHW机理能够在相关工艺条件(315-430℃)下高精度地反映低负载Ni/Al 2 O 3催化剂的动力学。 , 1-6 bar, H 2与 CO 2的摩尔比在 1-16 之间以及不同的试剂和产品分压)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号