当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introducing oxophilic metal and interstitial hydrogen into the Pd lattice to boost electrochemical performance for alkaline ethanol oxidation
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2021-12-18 , DOI: 10.1039/d1ta08924b Cong Shen 1 , Hanming Chen 1 , Mingye Qiu 1 , Yuqiang Shi 1 , Wei Yan 1 , Qiaorong Jiang 1 , Yaqi Jiang 1 , Zhaoxiong Xie 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2021-12-18 , DOI: 10.1039/d1ta08924b Cong Shen 1 , Hanming Chen 1 , Mingye Qiu 1 , Yuqiang Shi 1 , Wei Yan 1 , Qiaorong Jiang 1 , Yaqi Jiang 1 , Zhaoxiong Xie 1
Affiliation
|
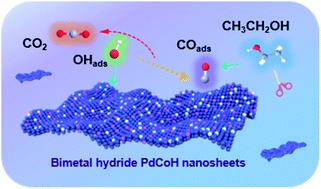
The sluggish kinetics of the ethanol oxidation reaction (EOR), poor C1 selectivity and susceptibility to toxicity of CO intermediates hinder the commercialization of direct ethanol fuel cells (DEFCs). In this paper, we reported an achievable method to fabricate an efficient catalyst of stable PdCoH nanosheets (NSs) for the EOR by introducing both the oxophilic metal cobalt and hydrogen atoms into the palladium lattice. Owing to the unique 2D curly sheet-structure of 2.3 nm thickness, the obtained catalyst exhibits a larger electrochemical active surface area. By comparison with PdCo NSs, PdH NSs, and commercial Pd/C counterparts, it was found that the obtained PdCoH NS catalyst exhibits the best electrocatalytic performance toward ethanol oxidation in alkaline solution, and its mass activity is higher than that of most of the Pd-based catalysts reported so far. The CO stripping and the in situ Fourier transform infrared (FTIR) spectroscopy study indicate that the incorporation of cobalt and hydrogen atoms is conducive to the improvement of anti-CO ability and the C1 pathway selectivity. Further investigation of the CV curves elucidates that the oxophilicity of Co in the PdCoH NSs is more favorable for the adsorption of OH−, which promotes the removal of COads. In addition, the valence band spectra show the lowest d-band center of the PdCoH NSs in comparison with PdCo NSs, PdH NSs, and commercial Pd/C counterparts, which helps weaken the adsorption of CO, and thus enhances the CO tolerance and C1 selectivity. We believe that this work opens the door for rational design of noble metal alloy catalysts with excellent performance through combining the advantages of oxophilic metals and H atoms.
中文翻译:

将亲氧金属和间隙氢引入 Pd 晶格以提高碱性乙醇氧化的电化学性能
乙醇氧化反应(EOR)动力学缓慢、C1选择性差和对CO中间体毒性的敏感性阻碍了直接乙醇燃料电池(DEFCs)的商业化。在本文中,我们报道了一种可实现的方法,通过将亲氧金属钴和氢原子引入钯晶格中来制造用于 EOR 的稳定 PdCoH 纳米片 (NSs) 的有效催化剂。由于独特的2.3 nm厚的二维卷曲片状结构,所获得的催化剂表现出更大的电化学活性表面积。通过与 PdCo NSs、PdH NSs 和商业 Pd/C 对应物的比较,发现所获得的 PdCoH NS 催化剂在碱性溶液中对乙醇氧化表现出最佳的电催化性能,并且其质量活性高于迄今为止报道的大多数Pd基催化剂。CO 汽提和原位傅里叶变换红外(FTIR)光谱研究表明,钴和氢原子的掺入有利于提高抗CO能力和C1途径选择性。的CV曲线阐明的是Co在PdCoH奈米球的oxophilicity为OH的吸附更有利的进一步调查- ,这促进去除CO的广告. 此外,与 PdCo NS、PdH NS 和商业 Pd/C 对应物相比,价带光谱显示 PdCoH NS 的 d 带中心最低,这有助于减弱 CO 的吸附,从而增强 CO 耐受性和 C1选择性。我们相信,这项工作通过结合亲氧金属和 H 原子的优势,为合理设计具有优异性能的贵金属合金催化剂打开了大门。
更新日期:2022-01-11
中文翻译:

将亲氧金属和间隙氢引入 Pd 晶格以提高碱性乙醇氧化的电化学性能
乙醇氧化反应(EOR)动力学缓慢、C1选择性差和对CO中间体毒性的敏感性阻碍了直接乙醇燃料电池(DEFCs)的商业化。在本文中,我们报道了一种可实现的方法,通过将亲氧金属钴和氢原子引入钯晶格中来制造用于 EOR 的稳定 PdCoH 纳米片 (NSs) 的有效催化剂。由于独特的2.3 nm厚的二维卷曲片状结构,所获得的催化剂表现出更大的电化学活性表面积。通过与 PdCo NSs、PdH NSs 和商业 Pd/C 对应物的比较,发现所获得的 PdCoH NS 催化剂在碱性溶液中对乙醇氧化表现出最佳的电催化性能,并且其质量活性高于迄今为止报道的大多数Pd基催化剂。CO 汽提和原位傅里叶变换红外(FTIR)光谱研究表明,钴和氢原子的掺入有利于提高抗CO能力和C1途径选择性。的CV曲线阐明的是Co在PdCoH奈米球的oxophilicity为OH的吸附更有利的进一步调查- ,这促进去除CO的广告. 此外,与 PdCo NS、PdH NS 和商业 Pd/C 对应物相比,价带光谱显示 PdCoH NS 的 d 带中心最低,这有助于减弱 CO 的吸附,从而增强 CO 耐受性和 C1选择性。我们相信,这项工作通过结合亲氧金属和 H 原子的优势,为合理设计具有优异性能的贵金属合金催化剂打开了大门。











































 京公网安备 11010802027423号
京公网安备 11010802027423号