当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A DFT study on the mechanism of HCl and CO2 capture by CaO
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-21 , DOI: 10.1039/d1re00487e Xiaotong Ma 1 , Xingkang Huang 1 , Tai Feng 1 , Mingfei Mu 1 , Xiude Hu 2
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-21 , DOI: 10.1039/d1re00487e Xiaotong Ma 1 , Xingkang Huang 1 , Tai Feng 1 , Mingfei Mu 1 , Xiude Hu 2
Affiliation
|
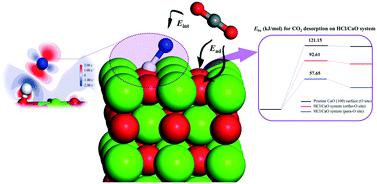
CaO-Based materials are considered to be feasible sorbents for the removal of both HCl and CO2. Theoretical calculations based on density functional theory (DFT) were performed to investigate the micro-mechanism of HCl/CO2 capture in a CaO-based chemical looping process. The results show that HCl preferentially interacts with surface O atoms on the CaO (100) surface, with a highest adsorption energy of −1.85 eV. The hydroxyl group, OH−, is observed during the adsorption process. At rising coverage, increased repulsion between the HCl molecules results in a lowering of the adsorption energy per HCl. The binding mechanisms of HCl and CO2 to CaO involve chemisorption and there is competing adsorption behavior between them due to positive interaction energy between coexisting HCl and CO2. The absolute values of the absorption energy and reaction barrier for CO2 adsorption and desorption in the HCl/CaO system are much lower than those on the pristine CaO (100) surface, which indicates the inhibition of CO2 adsorption and the promotion of CO2 desorption in the presence of HCl. With HCl adsorbed, the peaks for the p orbitals of the ortho-O and para-O atoms decrease by 13% and 5% in electron density, respectively, near to the Fermi level, and the ortho peak shifts from −1.79 to −0.45 eV; these aspects are inferred to play important roles in CO2 adsorption/desorption on CaO.
中文翻译:

CaO捕集HCl和CO2机理的DFT研究
CaO 基材料被认为是去除 HCl 和 CO 2 的可行吸附剂。进行了基于密度泛函理论 (DFT) 的理论计算,以研究基于 CaO 的化学循环过程中 HCl/CO 2捕获的微观机制。结果表明,HCl优先与CaO(100)表面上的表面O原子相互作用,最高吸附能为-1.85 eV。在吸附过程中观察到羟基,OH -。在增加覆盖率时,HCl 分子之间的排斥力增加导致每个 HCl 的吸附能降低。HCl和CO 2的结合机制对CaO 的吸附涉及化学吸附,由于共存的HCl 和CO 2之间存在正相互作用能,因此它们之间存在竞争吸附行为。吸收的能量和反应阻挡的用于CO绝对值2吸附和脱附的盐酸/ CaO的系统比在原始的CaO(100)表面,这表明CO的抑制低得多2吸附和促进CO的2在 HCl 存在下解吸。随着 HCl 的吸附,邻-O 和对-O 原子的 p 轨道峰的电子密度分别降低了 13% 和 5%,接近费米能级,而邻峰移从 -1.79 到 -0.45 eV;这些方面被推断在 CaO 上的CO 2吸附/解吸中起重要作用。
更新日期:2022-01-06
中文翻译:

CaO捕集HCl和CO2机理的DFT研究
CaO 基材料被认为是去除 HCl 和 CO 2 的可行吸附剂。进行了基于密度泛函理论 (DFT) 的理论计算,以研究基于 CaO 的化学循环过程中 HCl/CO 2捕获的微观机制。结果表明,HCl优先与CaO(100)表面上的表面O原子相互作用,最高吸附能为-1.85 eV。在吸附过程中观察到羟基,OH -。在增加覆盖率时,HCl 分子之间的排斥力增加导致每个 HCl 的吸附能降低。HCl和CO 2的结合机制对CaO 的吸附涉及化学吸附,由于共存的HCl 和CO 2之间存在正相互作用能,因此它们之间存在竞争吸附行为。吸收的能量和反应阻挡的用于CO绝对值2吸附和脱附的盐酸/ CaO的系统比在原始的CaO(100)表面,这表明CO的抑制低得多2吸附和促进CO的2在 HCl 存在下解吸。随着 HCl 的吸附,邻-O 和对-O 原子的 p 轨道峰的电子密度分别降低了 13% 和 5%,接近费米能级,而邻峰移从 -1.79 到 -0.45 eV;这些方面被推断在 CaO 上的CO 2吸附/解吸中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号