当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhaled Medicines: Past, Present, and Future
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-01-01 , DOI: 10.1124/pharmrev.120.000108 Sandra Anderson 1 , Paul Atkins 1 , Per Bäckman 1 , David Cipolla 1 , Andrew Clark 1 , Evangelia Daviskas 1 , Bernd Disse 1 , Plamena Entcheva-Dimitrov 1 , Rick Fuller 1 , Igor Gonda 2 , Hans Lundbäck 1 , Bo Olsson 1 , Jeffry Weers 1
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-01-01 , DOI: 10.1124/pharmrev.120.000108 Sandra Anderson 1 , Paul Atkins 1 , Per Bäckman 1 , David Cipolla 1 , Andrew Clark 1 , Evangelia Daviskas 1 , Bernd Disse 1 , Plamena Entcheva-Dimitrov 1 , Rick Fuller 1 , Igor Gonda 2 , Hans Lundbäck 1 , Bo Olsson 1 , Jeffry Weers 1
Affiliation
|
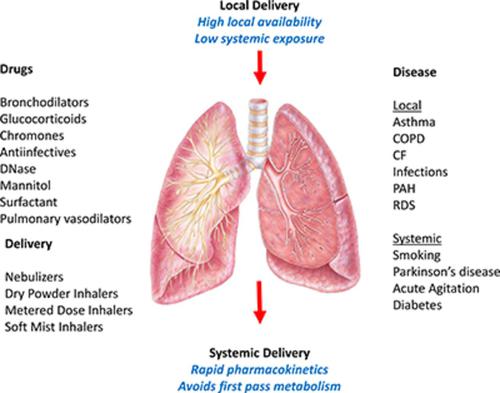
The purpose of this review is to summarize essential pharmacological, pharmaceutical, and clinical aspects in the field of orally inhaled therapies that may help scientists seeking to develop new products. After general comments on the rationale for inhaled therapies for respiratory disease, the focus is on products approved approximately over the last half a century. The organization of these sections reflects the key pharmacological categories. Products for asthma and chronic obstructive pulmonary disease include β-2 receptor agonists, muscarinic acetylcholine receptor antagonists, glucocorticosteroids, and cromones as well as their combinations. The antiviral and antibacterial inhaled products to treat respiratory tract infections are then presented. Two “mucoactive” products—dornase α and mannitol, which are both approved for patients with cystic fibrosis—are reviewed. These are followed by sections on inhaled prostacyclins for pulmonary arterial hypertension and the challenging field of aerosol surfactant inhalation delivery, especially for prematurely born infants on ventilation support. The approved products for systemic delivery via the lungs for diseases of the central nervous system and insulin for diabetes are also discussed. New technologies for drug delivery by inhalation are analyzed, with the emphasis on those that would likely yield significant improvements over the technologies in current use or would expand the range of drugs and diseases treatable by this route of administration.
中文翻译:

吸入药物:过去、现在和未来
本综述的目的是总结口服吸入疗法领域的基本药理学、药学和临床方面,可能有助于寻求开发新产品的科学家。在对呼吸系统疾病吸入疗法的基本原理进行一般性评论之后,重点是大约在过去半个世纪内获得批准的产品。这些部分的组织反映了关键的药理学类别。用于哮喘和慢性阻塞性肺病的产品包括β -2 受体激动剂、毒蕈碱乙酰胆碱受体拮抗剂、糖皮质激素和克罗蒙及其组合。然后介绍了治疗呼吸道感染的抗病毒和抗菌吸入产品。两种“粘液活性”产物——多核糖核酸酶α对都被批准用于囊性纤维化患者的甘露醇进行了审查。接下来是关于吸入前列环素治疗肺动脉高压的部分,以及气溶胶表面活性剂吸入给药的挑战性领域,特别是对于需要通气支持的早产儿。还讨论了经批准的用于通过肺部全身给药治疗中枢神经系统疾病和胰岛素治疗糖尿病的产品。分析了通过吸入给药的新技术,重点是那些可能对当前使用的技术产生重大改进或将扩大通过这种给药途径治疗的药物和疾病范围的技术。
更新日期:2022-01-06
中文翻译:

吸入药物:过去、现在和未来
本综述的目的是总结口服吸入疗法领域的基本药理学、药学和临床方面,可能有助于寻求开发新产品的科学家。在对呼吸系统疾病吸入疗法的基本原理进行一般性评论之后,重点是大约在过去半个世纪内获得批准的产品。这些部分的组织反映了关键的药理学类别。用于哮喘和慢性阻塞性肺病的产品包括β -2 受体激动剂、毒蕈碱乙酰胆碱受体拮抗剂、糖皮质激素和克罗蒙及其组合。然后介绍了治疗呼吸道感染的抗病毒和抗菌吸入产品。两种“粘液活性”产物——多核糖核酸酶α对都被批准用于囊性纤维化患者的甘露醇进行了审查。接下来是关于吸入前列环素治疗肺动脉高压的部分,以及气溶胶表面活性剂吸入给药的挑战性领域,特别是对于需要通气支持的早产儿。还讨论了经批准的用于通过肺部全身给药治疗中枢神经系统疾病和胰岛素治疗糖尿病的产品。分析了通过吸入给药的新技术,重点是那些可能对当前使用的技术产生重大改进或将扩大通过这种给药途径治疗的药物和疾病范围的技术。










































 京公网安备 11010802027423号
京公网安备 11010802027423号