当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expeditious base-free solid-state reaction between phenyl boronates and hydrogen peroxide on silica gel
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-13 , DOI: 10.1039/d1re00495f Li Zhang 1 , Qianqian Chen 1 , Li Yang 1 , Yining He 1 , Keke Guo 1 , Jialin Yang 1 , Ji-Min Han 1
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-13 , DOI: 10.1039/d1re00495f Li Zhang 1 , Qianqian Chen 1 , Li Yang 1 , Yining He 1 , Keke Guo 1 , Jialin Yang 1 , Ji-Min Han 1
Affiliation
|
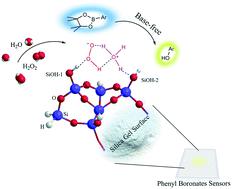
The conversion of phenyl boronates to phenol with hydrogen peroxide (H2O2) usually requires a base as a chemical assistant. In this work, we reported a base-free reaction between a phenyl boronate thin film and H2O2 vapor on a nanostructured silica gel surface and the yield is determined as 80% in 25 s. A fluorescent compound C6NIB was used to monitor the reaction process in both solution and the solid state. The fluorescent tests suggested that the silica gel surface can effectively promote the phenyl boronate compound reacting with H2O2 immediately on the solid state. Through control experiments, we determined the optimal reaction conditions. SEM, BET and water vapor adsorption characterization illustrated that the evenly distributed nanoscale pores and large surface areas of the silica gel interface may be critical for catalysis. Moreover, UV-vis absorption and reflection spectroscopy revealed that the final product of the base-free reaction was neutral phenol, instead of phenolate under basic conditions. Compared with traditional base-in reactions, the newly reported silica gel involved surface reaction could significantly extend the lifetime of the indicator molecules, resulting in excellent storage performance for practical sensing application. Finally, the simulation experiments identified the HO2−/H3O+ ionic pair on the interacting Si–OH surface group pairs on the silica surface as the key for catalysis. Thus, the generated HO2− then in turn reacted with phenyl boronate to form a phenol structure. The nanosized pores, arranged hydrogen bonding, and organized surface interface together led to this expeditious and effective base-free reaction between boronates and H2O2.
中文翻译:

硼酸苯酯与过氧化氢在硅胶上的快速无碱固态反应
使用过氧化氢 (H 2 O 2 ) 将硼酸苯酯转化为苯酚通常需要碱作为化学助剂。在这项工作中,我们报道了硼酸苯酯薄膜与纳米结构硅胶表面上的H 2 O 2蒸汽之间的无碱反应,产率在 25 秒内确定为 80%。荧光化合物 C6NIB 用于监测溶液和固态的反应过程。荧光测试表明硅胶表面能有效促进苯基硼酸酯化合物与H 2 O 2反应马上就固态了。通过对照实验,我们确定了最佳反应条件。SEM、BET 和水蒸气吸附表征表明,均匀分布的纳米级孔隙和硅胶界面的大表面积可能对催化作用至关重要。此外,紫外-可见吸收和反射光谱表明,无碱反应的最终产物是中性苯酚,而不是碱性条件下的苯酚盐。与传统的base-in反应相比,新报道的硅胶表面反应可以显着延长指示剂分子的寿命,从而为实际传感应用带来优异的存储性能。最后,模拟实验确定了 HO 2 − /H 3硅表面上相互作用的 Si-OH 表面基团上的O +离子对是催化的关键。因此,生成的HO 2 -然后又与硼酸苯酯反应形成苯酚结构。纳米尺寸的孔、排列的氢键和有序的表面界面共同导致硼酸盐和 H 2 O 2之间发生这种快速有效的无碱反应。
更新日期:2022-01-04
中文翻译:

硼酸苯酯与过氧化氢在硅胶上的快速无碱固态反应
使用过氧化氢 (H 2 O 2 ) 将硼酸苯酯转化为苯酚通常需要碱作为化学助剂。在这项工作中,我们报道了硼酸苯酯薄膜与纳米结构硅胶表面上的H 2 O 2蒸汽之间的无碱反应,产率在 25 秒内确定为 80%。荧光化合物 C6NIB 用于监测溶液和固态的反应过程。荧光测试表明硅胶表面能有效促进苯基硼酸酯化合物与H 2 O 2反应马上就固态了。通过对照实验,我们确定了最佳反应条件。SEM、BET 和水蒸气吸附表征表明,均匀分布的纳米级孔隙和硅胶界面的大表面积可能对催化作用至关重要。此外,紫外-可见吸收和反射光谱表明,无碱反应的最终产物是中性苯酚,而不是碱性条件下的苯酚盐。与传统的base-in反应相比,新报道的硅胶表面反应可以显着延长指示剂分子的寿命,从而为实际传感应用带来优异的存储性能。最后,模拟实验确定了 HO 2 − /H 3硅表面上相互作用的 Si-OH 表面基团上的O +离子对是催化的关键。因此,生成的HO 2 -然后又与硼酸苯酯反应形成苯酚结构。纳米尺寸的孔、排列的氢键和有序的表面界面共同导致硼酸盐和 H 2 O 2之间发生这种快速有效的无碱反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号