Gastroenterology ( IF 25.7 ) Pub Date : 2021-12-30 , DOI: 10.1053/j.gastro.2021.12.273 Abdel Nasser Hosein 1 , Gita Dangol 2 , Takashi Okumura 2 , Jason Roszik 3 , Kimal Rajapakshe 2 , Megan Siemann 2 , Mohamed Zaid 4 , Bidyut Ghosh 2 , Maria Monberg 2 , Paola A Guerrero 2 , Aatur Singhi 5 , Cara L Haymaker 2 , Hans Clevers 6 , Lotfi Abou-Elkacem 2 , Sonja M Woermann 2 , Anirban Maitra 2
|
Background & Aims
RNF43 is an E3 ubiquitin ligase that is recurrently mutated in pancreatic ductal adenocarcinoma (PDAC) and precursor cystic neoplasms of the pancreas. The impact of RNF43 mutations on PDAC is poorly understood and autochthonous models have not been characterized sufficiently. In this study, we describe a genetically engineered mouse model (GEMM) of PDAC with conditional expression of oncogenic Kras and deletion of the catalytic domain of Rnf43 in exocrine cells.
Methods
We generated Ptf1a-Cre;LSL-KrasG12D;Rnf43flox/flox (KRC) and Ptf1a-Cre; LSL-KrasG12D (KC) mice and animal survival was assessed. KRC mice were sacrificed at 2 months, 4 months, and at moribund status followed by analysis of pancreata by single-cell RNA sequencing. Comparative analyses between moribund KRC and a moribund Kras/Tp53-driven PDAC GEMM (KPC) was performed. Cell lines were isolated from KRC and KC tumors and interrogated by cytokine array analyses, ATAC sequencing, and in vitro drug assays. KRC GEMMs were also treated with an anti-CTLA4 neutralizing antibody with treatment response measured by magnetic response imaging.
Results
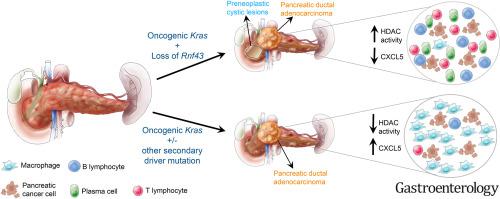
We demonstrate that KRC mice display a marked increase in incidence of high-grade cystic lesions of the pancreas and PDAC compared with KC. Importantly, KRC mice have a significantly decreased survival compared with KC mice. Using single-cell RNA sequencing, we demonstrated that KRC tumor progression is accompanied by a decrease in macrophages, as well as an increase in T and B lymphocytes, with evidence of increased immune checkpoint molecule expression and affinity maturation, respectively. This was in stark contrast to the tumor immune microenvironment observed in the KPC PDAC GEMM. Furthermore, expression of the chemokine CXCL5 was found to be specifically decreased in KRC cancer cells by means of epigenetic regulation and emerged as a putative candidate for mediating the unique KRC immune landscape.
Conclusions
The KRC GEMM establishes RNF43 as a bona fide tumor suppressor gene in PDAC. This GEMM features a markedly different immune microenvironment compared with previously reported PDAC GEMMs and puts forth a rationale for an immunotherapy approach in this subset of PDAC cases.
中文翻译:

Rnf43 的缺失会加速 Kras 介导的肿瘤形成并重塑胰腺癌中的肿瘤免疫微环境
背景与目标
RNF43是一种 E3 泛素连接酶,在胰腺导管腺癌 (PDAC) 和胰腺前体囊性肿瘤中反复突变。 RNF43突变对 PDAC 的影响知之甚少,并且本地模型尚未得到充分表征。在这项研究中,我们描述了 PDAC 的基因工程小鼠模型 (GEMM),该模型在外分泌细胞中条件性表达致癌Kras并删除Rnf43催化结构域。
方法
我们生成了Ptf1a-Cre; LSL-克拉斯G12D; Rnf43 flox/flox (KRC)和 Ptf1a-Cre;评估了 LSL- Kras G12D (KC) 小鼠和动物的存活率。在 2 个月、4 个月和垂死状态下处死 KRC 小鼠,然后通过单细胞 RNA 测序对胰腺进行分析。对垂死的 KRC 和垂死的Kras/Tp53驱动的 PDAC GEMM (KPC) 进行了比较分析。从 KRC 和 KC 肿瘤中分离细胞系,并通过细胞因子阵列分析、ATAC 测序和体外药物测定进行研究。 KRC GEMM 还用抗 CTLA4 中和抗体进行处理,并通过磁响应成像测量治疗反应。
结果
我们证明,与 KC 相比,KRC 小鼠胰腺和 PDAC 的高级囊性病变的发生率显着增加。重要的是,与 KC 小鼠相比,KRC 小鼠的存活率显着降低。使用单细胞 RNA 测序,我们证明 KRC 肿瘤进展伴随着巨噬细胞的减少以及 T 和 B 淋巴细胞的增加,有证据表明免疫检查点分子表达和亲和力成熟分别增加。这与 KPC PDAC GEMM 中观察到的肿瘤免疫微环境形成鲜明对比。此外,通过表观遗传调控,KRC 癌细胞中趋化因子 CXCL5 的表达明显降低,并成为介导独特 KRC 免疫景观的假定候选者。
结论
KRC GEMM 将RNF43确立为 PDAC 中真正的肿瘤抑制基因。与之前报道的 PDAC GEMM 相比,该 GEMM 具有明显不同的免疫微环境,并为该 PDAC 病例子集的免疫治疗方法提出了基本原理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号