当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Data Correlation of Pyrimethamine in 13 Pure Solvents at Temperatures from 278.15 to 318.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-30 , DOI: 10.1021/acs.jced.1c00672 Tong Song 1 , Yuntian Xiao 1 , Ketao Shi 1 , Yunhe Bai 1 , Jiulong Li 1 , Luguang Qi 1 , Chuang Xie 1, 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-30 , DOI: 10.1021/acs.jced.1c00672 Tong Song 1 , Yuntian Xiao 1 , Ketao Shi 1 , Yunhe Bai 1 , Jiulong Li 1 , Luguang Qi 1 , Chuang Xie 1, 2, 3
Affiliation
|
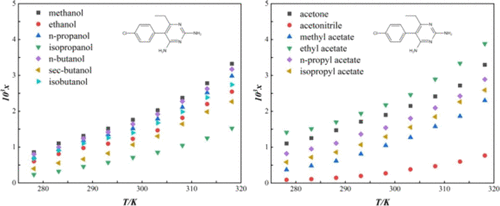
The solubility of PMN in 13 organic solvents (methanol, ethanol, n-propanol, isopropanol, n-butanol, sec-butanol, isobutanol, acetone, acetonitrile, methyl acetate, ethyl acetate, n-propyl acetate, and isopropyl acetate) was determined by the gravimetric method over the temperatures ranging from 278.15 to 318.15 K at 0.1 MPa. The solubility of all selected solvents increases with increasing temperature; however, the change in methanol is substantially more than that in other solvents. Four models, including the modified Apelblat equation, the λh equation, the NRTL, and the van’t Hoff equation, were applied to correlate the experimental solubility data, which showed that a relative deviation is less than 3% with the four models. In addition, a KAT-LSER model was employed to quantitatively evaluate and describe the solvent effect, which indicate that the hydrogen bonding basicity of solvents has a greater effect on the solubility of PMN.
中文翻译:

乙胺嘧啶在 278.15 至 318.15 K 温度下在 13 种纯溶剂中的溶解度测量和数据关联
测定了 PMN 在 13 种有机溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、异丁醇、丙酮、乙腈、乙酸甲酯、乙酸乙酯、乙酸正丙酯和乙酸异丙酯)中的溶解度通过重量法在 278.15 至 318.15 K、0.1 MPa 的温度范围内。所有选定溶剂的溶解度都随着温度的升高而增加;然而,甲醇的变化远大于其他溶剂的变化。四个模型,包括修改后的 Apelblat 方程、λ h应用方程、NRTL 和 van't Hoff 方程来关联实验溶解度数据,这表明四个模型的相对偏差小于 3%。此外,采用KAT-LSER模型对溶剂效应进行定量评价和描述,表明溶剂的氢键碱度对PMN溶解度的影响更大。
更新日期:2022-01-13
中文翻译:

乙胺嘧啶在 278.15 至 318.15 K 温度下在 13 种纯溶剂中的溶解度测量和数据关联
测定了 PMN 在 13 种有机溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、异丁醇、丙酮、乙腈、乙酸甲酯、乙酸乙酯、乙酸正丙酯和乙酸异丙酯)中的溶解度通过重量法在 278.15 至 318.15 K、0.1 MPa 的温度范围内。所有选定溶剂的溶解度都随着温度的升高而增加;然而,甲醇的变化远大于其他溶剂的变化。四个模型,包括修改后的 Apelblat 方程、λ h应用方程、NRTL 和 van't Hoff 方程来关联实验溶解度数据,这表明四个模型的相对偏差小于 3%。此外,采用KAT-LSER模型对溶剂效应进行定量评价和描述,表明溶剂的氢键碱度对PMN溶解度的影响更大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号