Gastroenterology ( IF 25.7 ) Pub Date : 2021-12-29 , DOI: 10.1053/j.gastro.2021.12.272 Wenjie Liang 1 , Emmanuelle Enée 1 , Cédric Andre-Vallee 1 , Marika Falcone 2 , Jia Sun 3 , Julien Diana 1
|
Background & Aims
Alteration of the gut microbiota is implicated in the development of autoimmune type 1 diabetes (T1D), as shown in humans and the nonobese diabetic (NOD) mouse model. However, how gut dysbiosis arises and promotes the autoimmune response remains an open question. We investigated whether early events affecting the intestinal homeostasis in newborn NOD mice may explain the development of the autoimmune response in the adult pancreas.
Methods
We profiled the transcriptome and the microbiota in the colon between newborn NOD mice and nonautoimmune strains. We identified a seminal defect in the intestinal homeostasis of newborn NOD mice and deciphered the mechanism linking this defect to the diabetogenic response in the adult.
Results
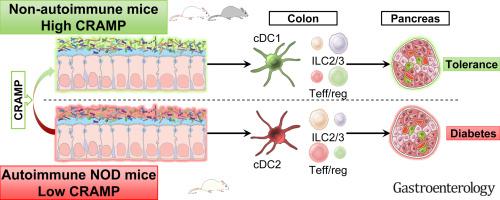
We determined that the cathelicidin-related antimicrobial peptide (CRAMP) expression was defective in the colon of newborn NOD mice, allowing inducing dysbiosis. Dysbiosis stimulated the colonic epithelial cells to produce type I interferons that pathologically imprinted the local neonatal immune system. This pathological immune imprinting later promoted the pancreatic autoimmune response in the adult and the development of diabetes. Increasing colonic CRAMP expression in newborn NOD mice by means of local CRAMP treatment or CRAMP-expressing probiotic restored colonic homeostasis and halted the diabetogenic response, preventing autoimmune diabetes.
Conclusions
We identified whether a defective colonic expression in the CRAMP antimicrobial peptide induces dysbiosis, contributing to autoimmunity in the pancreas. Hence, the manipulation of intestinal antimicrobial peptides may be considered a relevant therapeutic approach to prevent autoimmune diabetes in at-risk children.
中文翻译:

肠道 Cathelicidin 抗菌肽可形成保护性新生儿肠道微生物群以对抗胰腺自身免疫
背景与目标
如人类和非肥胖糖尿病 (NOD) 小鼠模型所示,肠道微生物群的改变与自身免疫性 1 型糖尿病 (T1D) 的发展有关。然而,肠道菌群失调如何产生并促进自身免疫反应仍然是一个悬而未决的问题。我们研究了影响新生 NOD 小鼠肠道稳态的早期事件是否可以解释成年胰腺中自身免疫反应的发展。
方法
我们分析了新生 NOD 小鼠和非自身免疫菌株之间结肠中的转录组和微生物群。我们确定了新生 NOD 小鼠肠道稳态的精液缺陷,并破译了将这种缺陷与成年糖尿病反应联系起来的机制。
结果
我们确定新生 NOD 小鼠结肠中的导管素相关抗菌肽 (CRAMP) 表达存在缺陷,从而导致生态失调。生态失调刺激结肠上皮细胞产生 I 型干扰素,这些干扰素在病理上印在新生儿局部免疫系统上。这种病理性免疫印记后来促进了成人胰腺自身免疫反应和糖尿病的发展。通过局部 CRAMP 治疗或表达 CRAMP 的益生菌增加新生 NOD 小鼠的结肠 CRAMP 表达可恢复结肠稳态并停止糖尿病反应,从而预防自身免疫性糖尿病。
结论
我们确定了 CRAMP 抗菌肽中的结肠表达缺陷是否会诱导生态失调,从而导致胰腺中的自身免疫。因此,肠道抗菌肽的操作可能被认为是预防高危儿童自身免疫性糖尿病的相关治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号