当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminocatalytic stereoselective synthesis of (E)-α-naphthyl enals via cross-coupling-like reaction of 1-bromo-2-naphthols with enals
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2021-11-1 , DOI: 10.1016/j.gresc.2021.08.001 Xixi Song , Fangchen Song , Xiang Meng , Peng Ji , Wei Wang
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2021-11-1 , DOI: 10.1016/j.gresc.2021.08.001 Xixi Song , Fangchen Song , Xiang Meng , Peng Ji , Wei Wang
|
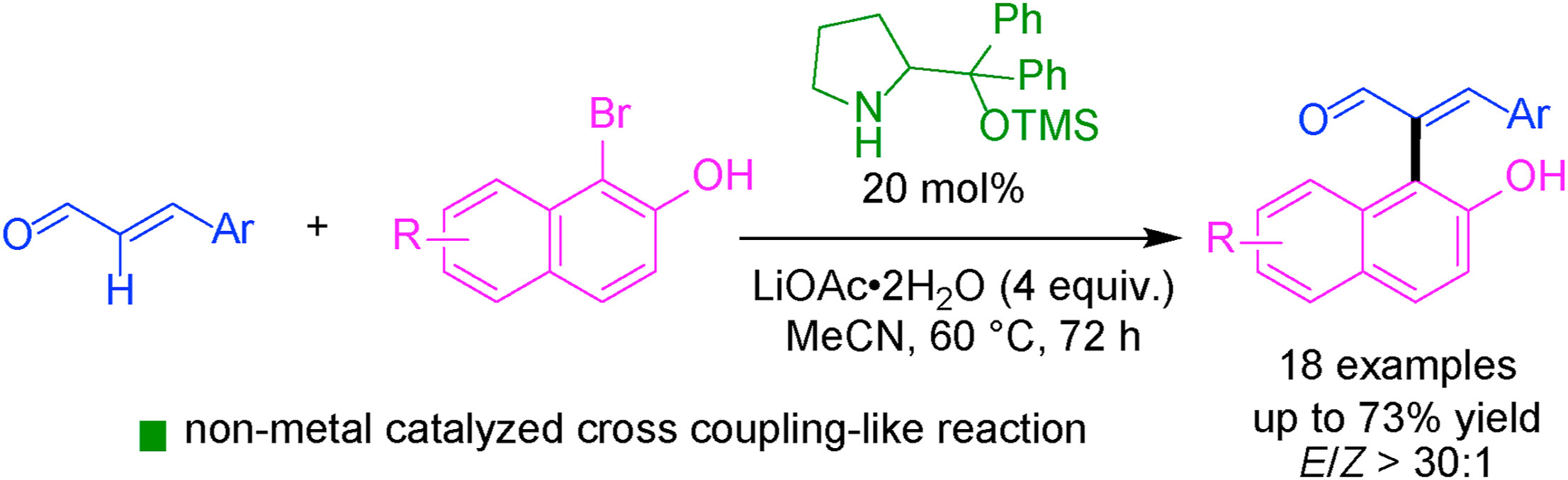
A diphenyl prolinol TMS ether-catalyzed stereoselective reaction of 1-bromo-2-naphthols with enals has been developed. The organocatalyzed cross coupling-like reaction servers an efficient approach to sterically congested (E)-α-naphthyl enals. The formal C(sp2)-C(sp2) cross-coupling products are obtained in good yields and with excellent stereoselectivities in favor of E-isomers. A broad range of aromatic enals and 1-bromo-2-naphthols are tolerated. The synthetic strategy significantly expands the scope of the organocatalytic cross-coupling-like reactions by enabling sterically demanding 1-bromo-2-naphthols as viable substrates.
中文翻译:

1-溴-2-萘酚与烯醛类交叉偶联反应的氨基催化立体选择性合成 (E)-α-萘烯醛
开发了二苯基脯氨醇 TMS 醚催化的 1-溴-2-萘酚与烯醛的立体选择性反应。有机催化的类交叉偶联反应为空间拥挤的 (E)-α-萘烯提供了一种有效的方法。正式的 C(sp2)-C(sp2) 交叉偶联产物以良好的收率获得,并且具有极好的立体选择性,有利于 E 异构体。可耐受范围广泛的芳香烯醛和 1-溴-2-萘酚。该合成策略通过使空间要求高的 1-溴-2-萘酚作为可行的底物,显着扩大了有机催化交叉偶联反应的范围。
更新日期:2022-01-13
中文翻译:

1-溴-2-萘酚与烯醛类交叉偶联反应的氨基催化立体选择性合成 (E)-α-萘烯醛
开发了二苯基脯氨醇 TMS 醚催化的 1-溴-2-萘酚与烯醛的立体选择性反应。有机催化的类交叉偶联反应为空间拥挤的 (E)-α-萘烯提供了一种有效的方法。正式的 C(sp2)-C(sp2) 交叉偶联产物以良好的收率获得,并且具有极好的立体选择性,有利于 E 异构体。可耐受范围广泛的芳香烯醛和 1-溴-2-萘酚。该合成策略通过使空间要求高的 1-溴-2-萘酚作为可行的底物,显着扩大了有机催化交叉偶联反应的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号