Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-12-25 , DOI: 10.1016/j.jhep.2021.12.019 Amanda Garrido 1 , Eunjeong Kim 1 , Ana Teijeiro 1 , Paula Sánchez Sánchez 1 , Rosa Gallo 1 , Ajay Nair 2 , María Matamala Montoya 1 , Cristian Perna 3 , Guillermo P Vicent 4 , Javier Muñoz 5 , Ramón Campos-Olivas 6 , Johannes C Melms 7 , Benjamin Izar 7 , Robert F Schwabe 2 , Nabil Djouder 1
|
Background & Aims
Owing to the lack of genetic animal models that adequately recreate key clinical characteristics of cirrhosis, the molecular pathogenesis of cirrhosis has been poorly characterized, and treatments remain limited. Hence, we aimed to better elucidate the pathological mechanisms of cirrhosis using a novel murine model.
Methods
We report on the first murine genetic model mimicking human cirrhosis induced by hepatocyte-specific elimination of microspherule protein 1 (MCRS1), a member of non-specific lethal (NSL) and INO80 chromatin-modifier complexes. Using this genetic tool with other mouse models, cell culture and human samples, combined with quantitative proteomics, single nuclei/cell RNA sequencing and chromatin immunoprecipitation assays, we investigated mechanisms of cirrhosis.
Results
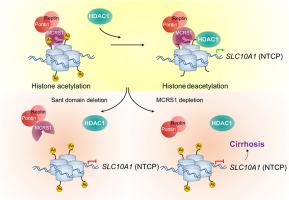
MCRS1 loss in mouse hepatocytes modulates the expression of bile acid (BA) transporters – with a pronounced downregulation of Na+-taurocholate cotransporting polypeptide (NTCP) – concentrating BAs in sinusoids and thereby activating hepatic stellate cells (HSCs) via the farnesoid X receptor (FXR), which is predominantly expressed in human and mouse HSCs. Consistently, re-expression of NTCP in mice reduces cirrhosis, and genetic ablation of FXR in HSCs suppresses fibrotic marks in mice and in vitro cell culture. Mechanistically, deletion of a putative SANT domain from MCRS1 evicts histone deacetylase 1 from its histone H3 anchoring sites, increasing histone acetylation of BA transporter genes, modulating their expression and perturbing BA flow. Accordingly, human cirrhosis displays decreased nuclear MCRS1 and NTCP expression.
Conclusions
Our data reveal a previously unrecognized function of MCRS1 as a critical histone acetylation regulator, maintaining gene expression and liver homeostasis. MCRS1 loss induces acetylation of BA transporter genes, perturbation of BA flow, and consequently, FXR activation in HSCs. This axis represents a central and universal signaling event in cirrhosis, which has significant implications for cirrhosis treatment.
Lay summary
By genetic ablation of MCRS1 in mouse hepatocytes, we generate the first genetic mouse model of cirrhosis that recapitulates human features. Herein, we demonstrate that the activation of the bile acid/FXR axis in liver fibroblasts is key in cirrhosis development.
中文翻译:

胆汁酸转运蛋白基因的组蛋白乙酰化在肝硬化中起关键作用
背景与目标
由于缺乏能够充分再现肝硬化关键临床特征的遗传动物模型,肝硬化的分子发病机制一直没有得到很好的表征,治疗仍然有限。因此,我们旨在使用一种新的小鼠模型更好地阐明肝硬化的病理机制。
方法
我们报告了第一个模拟人类肝硬化的小鼠遗传模型,该模型由肝细胞特异性消除微球蛋白 1 (MCRS1)、非特异性致死 (NSL) 和 INO80 染色质修饰复合物的成员引起。我们将这种遗传工具与其他小鼠模型、细胞培养物和人体样本结合使用,结合定量蛋白质组学、单核/细胞 RNA 测序和染色质免疫沉淀测定,研究了肝硬化的机制。
结果
小鼠肝细胞中 MCRS1 的缺失会调节胆汁酸 (BA) 转运蛋白的表达——明显下调 Na + -牛磺胆酸协同转运多肽 (NTCP)——将 BAs 浓缩在血窦中,从而通过法尼醇 X 受体激活肝星状细胞 (HSC)。 FXR),主要在人和小鼠 HSC 中表达。一致地,小鼠体内 NTCP 的重新表达可减少肝硬化,HSC 中FXR的基因消融可抑制小鼠和体外的纤维化标记细胞培养。从机制上讲,从 MCRS1 中删除假定的 SANT 结构域会将组蛋白去乙酰化酶 1 从其组蛋白 H3 锚定位点驱逐,增加 BA 转运蛋白基因的组蛋白乙酰化,调节它们的表达并扰乱 BA 流动。因此,人类肝硬化显示核 MCRS1 和 NTCP 表达减少。
结论
我们的数据揭示了 MCRS1 作为一种关键的组蛋白乙酰化调节剂,维持基因表达和肝脏稳态的先前未被认识的功能。MCRS1 丢失诱导 BA 转运蛋白基因的乙酰化、BA 流动的扰动,从而导致 HSC 中的 FXR 激活。该轴代表肝硬化中的中心和普遍信号事件,对肝硬化治疗具有重要意义。
总结
通过小鼠肝细胞中 MCRS1 的基因消融,我们生成了第一个概括人类特征的肝硬化遗传小鼠模型。在此,我们证明肝成纤维细胞中胆汁酸/FXR 轴的激活是肝硬化发展的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号