当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Continuous synthesis of Cu/ZnO/Al2O3 nanoparticles in a co-precipitation reaction using a silicon based microfluidic reactor
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-17 , DOI: 10.1039/d1re00499a Ghazal Tofighi 1 , Henning Lichtenberg 1, 2 , Abhijeet Gaur 1, 2 , Wu Wang 3 , Stefan Wild 2 , Karla Herrera Delgado 2 , Stephan Pitter 2 , Roland Dittmeyer 4 , Jan-Dierk Grunwaldt 1, 2 , Dmitry E. Doronkin 1, 2
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2021-12-17 , DOI: 10.1039/d1re00499a Ghazal Tofighi 1 , Henning Lichtenberg 1, 2 , Abhijeet Gaur 1, 2 , Wu Wang 3 , Stefan Wild 2 , Karla Herrera Delgado 2 , Stephan Pitter 2 , Roland Dittmeyer 4 , Jan-Dierk Grunwaldt 1, 2 , Dmitry E. Doronkin 1, 2
Affiliation
|
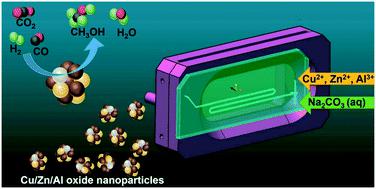
CuO/ZnO/Al2O3 catalysts were continuously synthesized in a microfluidic reactor, analyzed by X-ray diffraction (XRD), physisorption (BET), chemisorption, electron microscopy and X-ray absorption spectroscopy (XAS), and tested for methanol synthesis from CO-rich synthesis gas. The results were compared to those obtained from CuO/ZnO and CuO/ZnO/Al2O3 produced by conventional co-precipitation in a batch reactor. The predominant phase of the aged precursor from microfluidic co-precipitation was identified as zincian malachite. After calcination the microfluidically synthesized catalyst exhibited smaller CuO crystallites, a larger BET surface area, a rather uniform morphology and a homogeneous distribution of Cu and Zn compared to catalysts prepared by batch co-precipitation. H2-Temperature programmed reduction (TPR) showed that Cu species in CuO/ZnO/Al2O3 from microfluidic co-precipitation were more easily reducible. In situ Cu and Zn K-edge XAS during the TPR indicated reduction of Cu2+ to Cu0 between 150 °C and 240 °C, without detectable reduction of Zn. N2O pulse chemisorption evidenced an enlarged Cu surface area of the nanoparticles from the microfluidic synthesis. Based on activity tests in methanol synthesis, at 250 °C the microfluidically synthesized Cu/ZnO/Al2O3 catalysts showed better performance than the catalyst from batch preparation when 1 mol% CO2 was present in the synthesis gas. Dimethyl ether formed as a side product. As the microreactor is specially designed for high X-ray transmission with a thin Si/glass observation window, this study opens interesting perspectives for investigating the formation of catalyst precursors at the early stage of precipitation in future.
中文翻译:

使用硅基微流体反应器在共沉淀反应中连续合成 Cu/ZnO/Al2O3 纳米颗粒
CuO/ZnO/Al 2 O 3催化剂在微流控反应器中连续合成,通过 X 射线衍射 (XRD)、物理吸附 (BET)、化学吸附、电子显微镜和 X 射线吸收光谱 (XAS) 分析,并测试甲醇由富含 CO 的合成气合成。将结果与从 CuO/ZnO 和 CuO/ZnO/Al 2 O 3获得的结果进行比较在间歇式反应器中通过常规共沉淀法生产。来自微流体共沉淀的老化前体的主要相被确定为锌孔雀石。与通过分批共沉淀制备的催化剂相比,煅烧后微流体合成的催化剂表现出更小的 CuO 微晶、更大的 BET 表面积、相当均匀的形态以及 Cu 和 Zn 的均匀分布。H 2 -程序升温还原(TPR) 表明来自微流体共沉淀的CuO/ZnO/Al 2 O 3中的Cu 物质更容易还原。TPR期间的原位Cu和Zn K-edge XAS表明Cu 2+还原为Cu 0在 150 °C 和 240 °C 之间,没有检测到 Zn 的减少。N 2 O 脉冲化学吸附证明来自微流体合成的纳米颗粒的铜表面积扩大。根据甲醇合成中的活性测试,在 250 °C 下,当合成气中存在1 mol% CO 2时,微流体合成的 Cu/ZnO/Al 2 O 3催化剂表现出比批量制备的催化剂更好的性能。二甲醚作为副产物形成。由于微反应器是专为高 X 射线透射率而设计的,具有薄的 Si/玻璃观察窗,这项研究为研究未来沉淀早期催化剂前体的形成开辟了有趣的前景。
更新日期:2021-12-24
中文翻译:

使用硅基微流体反应器在共沉淀反应中连续合成 Cu/ZnO/Al2O3 纳米颗粒
CuO/ZnO/Al 2 O 3催化剂在微流控反应器中连续合成,通过 X 射线衍射 (XRD)、物理吸附 (BET)、化学吸附、电子显微镜和 X 射线吸收光谱 (XAS) 分析,并测试甲醇由富含 CO 的合成气合成。将结果与从 CuO/ZnO 和 CuO/ZnO/Al 2 O 3获得的结果进行比较在间歇式反应器中通过常规共沉淀法生产。来自微流体共沉淀的老化前体的主要相被确定为锌孔雀石。与通过分批共沉淀制备的催化剂相比,煅烧后微流体合成的催化剂表现出更小的 CuO 微晶、更大的 BET 表面积、相当均匀的形态以及 Cu 和 Zn 的均匀分布。H 2 -程序升温还原(TPR) 表明来自微流体共沉淀的CuO/ZnO/Al 2 O 3中的Cu 物质更容易还原。TPR期间的原位Cu和Zn K-edge XAS表明Cu 2+还原为Cu 0在 150 °C 和 240 °C 之间,没有检测到 Zn 的减少。N 2 O 脉冲化学吸附证明来自微流体合成的纳米颗粒的铜表面积扩大。根据甲醇合成中的活性测试,在 250 °C 下,当合成气中存在1 mol% CO 2时,微流体合成的 Cu/ZnO/Al 2 O 3催化剂表现出比批量制备的催化剂更好的性能。二甲醚作为副产物形成。由于微反应器是专为高 X 射线透射率而设计的,具有薄的 Si/玻璃观察窗,这项研究为研究未来沉淀早期催化剂前体的形成开辟了有趣的前景。










































 京公网安备 11010802027423号
京公网安备 11010802027423号