当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Correlation of the Ca2+ and Mg2+ Solubility in Ammonium Polyphosphate Solution from 278.2 to 323.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-24 , DOI: 10.1021/acs.jced.1c00830 Yumei Wang 1 , Dehua Xu 1 , Jingxu Yang 1 , Zhengjuan Yan 1 , Tao Luo 1 , Xue Li 1 , Zhiye Zhang 1 , Xinlong Wang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-24 , DOI: 10.1021/acs.jced.1c00830 Yumei Wang 1 , Dehua Xu 1 , Jingxu Yang 1 , Zhengjuan Yan 1 , Tao Luo 1 , Xue Li 1 , Zhiye Zhang 1 , Xinlong Wang 1
Affiliation
|
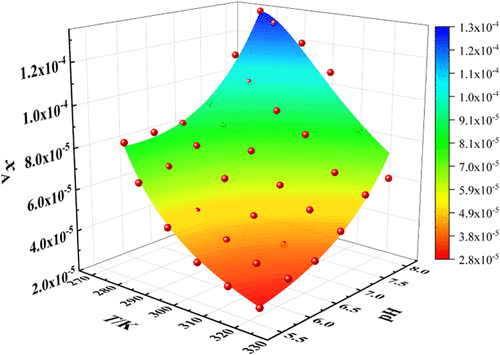
The solubility data of Ca2+ and Mg2+ in ammonium polyphosphate (APP) solution are the key to the formulation of fluid fertilizers, especially in areas where hard water with excessive Ca2+ and Mg2+ is conventionally used for irrigation. The solubility of Ca2+ and Mg2+ in APP solutions of four different Mg2+/Ca2+ molar ratios is determined by the dynamic method at the temperature ranging from 278.2 to 323.2 K and at solution pH values ranging from 5.5 to 8.0 under an atmospheric pressure. The X-ray diffraction patterns of the solid phase formed in the four abovementioned types of mixed solutions showed that their crystal structures are different and that the crystal structure conversion occurs in the process of dissolution and crystallization. The solubility of Ca2+ and Mg2+ increased with the ascending temperature and the solution pH values. The solubility of Ca2+ in APP solutions decreased with the increase of the Mg2+ molar ratio [x(Mg2+)] relative to the total Ca2+ and Mg2+ amount, while the solubility of Mg2+ increased with the increase of x(Mg2+). The experimental solubility data, expressed in molar ratios of components, were correlated with the modified Apelblat equation. The correlation showed good agreement with the experimental results, and the average values of the root-mean-square deviation were 1.46 × 10–2 for Ca2+ and 4.58 × 10–2 for Mg2+, respectively.
中文翻译:

278.2 至 323.2 K 聚磷酸铵溶液中 Ca2+ 和 Mg2+ 溶解度的测定及其相关性
Ca 2+和 Mg 2+在聚磷酸铵 (APP) 溶液中的溶解度数据是配制液体肥料的关键,特别是在通常使用含有过量 Ca 2+和 Mg 2+的硬水进行灌溉的地区。Ca 2+和Mg 2+在四种不同Mg 2+ /Ca 2+的APP溶液中的溶解度摩尔比是通过动态方法在 278.2 至 323.2 K 的温度和 5.5 至 8.0 的溶液 pH 值在大气压下确定的。上述四种混合溶液中形成的固相的X射线衍射图谱表明,它们的晶体结构不同,在溶解和结晶过程中发生了晶体结构的转换。Ca 2+和Mg 2+的溶解度随着温度和溶液pH值的升高而增加。APP溶液中Ca 2+的溶解度随着Mg 2+摩尔比[ x (Mg 2+ )]相对于总Ca 2+的增加而降低和Mg 2+量,而Mg 2+ 的溶解度随着x (Mg 2+ )的增加而增加。以组分摩尔比表示的实验溶解度数据与改进的 Apelblat 方程相关。相关性与实验结果吻合较好,Ca 2+的均方根偏差平均值分别为1.46 × 10 -2和Mg 2+的4.58 × 10 -2。
更新日期:2022-01-13
中文翻译:

278.2 至 323.2 K 聚磷酸铵溶液中 Ca2+ 和 Mg2+ 溶解度的测定及其相关性
Ca 2+和 Mg 2+在聚磷酸铵 (APP) 溶液中的溶解度数据是配制液体肥料的关键,特别是在通常使用含有过量 Ca 2+和 Mg 2+的硬水进行灌溉的地区。Ca 2+和Mg 2+在四种不同Mg 2+ /Ca 2+的APP溶液中的溶解度摩尔比是通过动态方法在 278.2 至 323.2 K 的温度和 5.5 至 8.0 的溶液 pH 值在大气压下确定的。上述四种混合溶液中形成的固相的X射线衍射图谱表明,它们的晶体结构不同,在溶解和结晶过程中发生了晶体结构的转换。Ca 2+和Mg 2+的溶解度随着温度和溶液pH值的升高而增加。APP溶液中Ca 2+的溶解度随着Mg 2+摩尔比[ x (Mg 2+ )]相对于总Ca 2+的增加而降低和Mg 2+量,而Mg 2+ 的溶解度随着x (Mg 2+ )的增加而增加。以组分摩尔比表示的实验溶解度数据与改进的 Apelblat 方程相关。相关性与实验结果吻合较好,Ca 2+的均方根偏差平均值分别为1.46 × 10 -2和Mg 2+的4.58 × 10 -2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号