Frontiers of Chemical Science and Engineering ( IF 4.3 ) Pub Date : 2021-12-22 , DOI: 10.1007/s11705-021-2111-5 Fateme Abbasi 1 , Zeinab Abbasi 1 , Cyrus Ghotbi 1 , Javad Karimi-Sabet 2
|
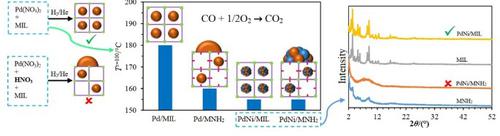
The solubility of Pd(NO3)2 in water is moderate whereas it is completely soluble in diluted HNO3 solution. Pd/MIL-101(Cr) and Pd/MIL-101-NH2(Cr) were synthesized by aqueous solution of Pd(NO3)2 and Pd(NO3)2 solution in dilute HNO3 and used for CO oxidation reaction. The catalysts synthesized with Pd(NO3)2 solution in dilute HNO3 showed lower activity. The aqueous solution of Pd(NO3)2 was used for synthesis of mono-metal Ni, Pd and bimetallic PdNi nanoparticles with various molar ratios supported on MOF. Pd70Ni30/MIL-101(Cr) catalyst showed higher activity than monometallic counterparts and Pd + Ni physical mixture due to the strong synergistic effect of PdNi nanoparticles, high distribution of PdNi nanoparticles, and lower dissociation and desorption barriers. Comparison of the catalysts synthesized by MIL-101(Cr) and MIL-101-NH2(Cr) as the supports of metals showed that Pd/MIL-101-NH2(Cr) outperforms Pd/MIL-101-(Cr) because of the higher electron density of Pd resulting from the electron donor ability of the NH2 functional group. However, the same activities were observed for Pd70Ni30/MIL-101(Cr) and Pd70Ni30/MIL-101-NH2(Cr), which is due to a less uniform distribution of Pd nanoparticles in Pd70Ni30/MIL-101-NH2(Cr) originated from amorphization of MIL-101-NH2(Cr) structure during the reduction process. In contrast, Pd70Ni30/MIL-101(Cr) revealed the stable structure and activity during reduction and CO oxidation for a long time.
中文翻译:

金属-有机骨架上负载的高活性和稳定的 PdNi 纳米粒子将 CO 转化为 CO2
Pd(NO 3 ) 2在水中的溶解度适中,而在稀HNO 3溶液中完全溶解。Pd/MIL-101(Cr)和Pd/MIL-101-NH 2 (Cr)由Pd(NO 3 ) 2和Pd(NO 3 ) 2稀HNO 3水溶液合成,用于CO氧化反应. 用稀HNO 3 中的Pd(NO 3 ) 2溶液合成的催化剂显示出较低的活性。Pd(NO 3 ) 2的水溶液用于合成单金属 Ni、Pd 和双金属 PdNi 纳米粒子,具有不同的摩尔比支持在 MOF 上。Pd 70 Ni 30 /MIL-101(Cr) 催化剂表现出比单金属对应物和 Pd + Ni 物理混合物更高的活性,这是由于 PdNi 纳米粒子的强协同作用、PdNi 纳米粒子的高分布以及较低的解离和解吸势垒。MIL-101(Cr)和MIL-101-NH 2 (Cr)作为金属载体合成的催化剂的比较表明Pd/MIL-101-NH 2 (Cr)优于Pd/MIL-101-(Cr)因为 NH 2官能团的电子供体能力导致 Pd 的电子密度更高。然而,对 Pd 观察到相同的活性70 Ni 30 /MIL-101(Cr) 和 Pd 70 Ni 30 /MIL-101-NH 2 (Cr),这是由于 Pd 纳米粒子在 Pd 70 Ni 30 /MIL-101-NH 2 中的分布不太均匀( Cr) 源于还原过程中 MIL-101-NH 2 (Cr) 结构的非晶化。相比之下,Pd 70 Ni 30 /MIL-101(Cr) 在还原和 CO 氧化过程中长时间显示出稳定的结构和活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号