Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2022-01-31 , DOI: 10.2174/1573412917666210121151724 Suhair S. Al-Nimry 1 , Khouloud A. Alkhamis 1 , Bashar M. Altaani 1
|
Background: Omeprazole has poor water solubility, is unstable in acidic solutions and undergoes first-pass metabolism, which results in lowering its bioavailability. A solid Self-Nano Emulsifying Drug Delivery System (SNEDDS) was previously prepared to enhance its dissolution.
Objective: Development and validation of an RP-HPLC method with UV detection for the determination of omeprazole in 0.1 N HCl and in 0.01 M phosphate buffer (pH 7.4).
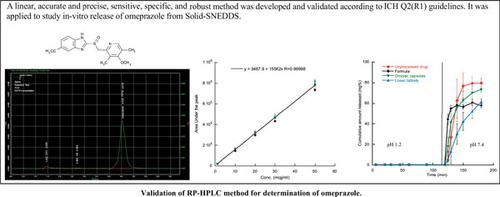
Methods: Validation was according to the ICH Q2 (R1) guidelines in terms of linearity, accuracy and precision, lower limit of quantification, sensitivity, specificity, and robustness. The developed and validated method was used to study the in-vitro dissolution of the drug from the solid- SNEDDS, commercial products, and the unprocessed drug. The dissolution was studied in 500 ml of 0.1N HCl during the first 2 hours, and 900 mL of 0.01 M phosphate buffer (pH 7.4) during the last hour (37 ± 0.5 °C and 100 rpm).
Results: The method was linear in the range 1-50 μg/ml, accurate and precise as indicated by the ANOVA test. It was specific to the drug and the pharmaceutical excipients did not affect the determination of its concentration. The method was robust to small changes in pH, composition, and flow rate of the mobile phase. The dissolution rate of omeprazole from the Solid-SNEDDS was faster than that from two commercial dosage forms and than the dissolution rate of the unprocessed drug.
Conclusion: The method met the acceptance criteria and was applied successfully in studying the rate of dissolution of the drug.
中文翻译:

RP-HPLC 法测定溶出介质中奥美拉唑的验证及其在固体-SNEDDS 体外释放研究中的应用
背景:奥美拉唑水溶性差,在酸性溶液中不稳定,并进行首过代谢,导致其生物利用度降低。先前制备了固体自纳米乳化药物递送系统 (SNEDDS) 以提高其溶出度。
目的:开发和验证具有紫外检测功能的 RP-HPLC 方法,用于测定 0.1 N HCl 和 0.01 M 磷酸盐缓冲液 (pH 7.4) 中的奥美拉唑。
方法:根据 ICH Q2 (R1) 指南在线性、准确度和精密度、定量下限、灵敏度、特异性和稳健性方面进行验证。开发和验证的方法用于研究药物从固体 SNEDDS、商业产品和未加工药物中的体外溶出度。在前 2 小时内在 500 ml 0.1N HCl 中研究溶解情况,在最后一个小时内(37 ± 0.5 °C 和 100 rpm)在 900 mL 0.01 M 磷酸盐缓冲液(pH 7.4)中进行研究。
结果:该方法在1-50 μg/ml范围内呈线性,ANOVA检验表明该方法准确、精密。它对药物具有特异性,药用辅料不影响其浓度的测定。该方法对流动相的 pH、组成和流速的微小变化具有稳健性。Solid-SNEDDS 中奥美拉唑的溶出速率快于两种商业剂型和未加工药物的溶出速率。
结论:该方法符合验收标准,成功应用于药物溶出度研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号