Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2022-01-31 , DOI: 10.2174/1573412917666210210141449 Asmaa Ahmed El Zaher 1 , Ehab Farouk El Kady 1 , Hind Ezzat El Ghwas 2 , Ola Mohamed El Houssini 2
|
Background: Design of experiment (DOE) is considered the most powerful tool to identify factors that affect variation and improve the response by tuning these factors. In the present work, DOE was applied to establish an innovative, sensitive, and precise HPLC method for the simultaneous determination of Escitalopram oxalate, Paroxetine hydrochloride hemihydrate and Clonazepam in the presence of their related compounds in drug substance and drug products.
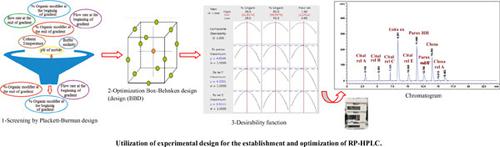
Methods: Buffer molarity, % organic modifier (acetonitrile content) at the beginning/end of the gradient, flow rate at the beginning/end of the gradient, pH of the mobile phase, and column temperature were screened using Plackett-Burman design (PBD) model. The main effect plot showed that % organic modifier at the beginning/end of gradient and flow rate at the beginning of gradient were statistically significant variables influencing peaks resolution (p<0.05). Box-Behnken design (BBD) was then used as an optimization model in order to achieve the highest possible resolution with the least possible experimental trials through studying the interaction and quadratic effects of these three factors. Finally, the optimum condition for predicated peak resolution could be achieved by the desirability function.
Results: After optimization, the chromatographic separation was attained on Agilent Zorbax SB C18 (4.6×250mm, 5μm) column using gradient elution of mobile phase: (A) potassium dihydrogen phosphate (pH 2.7; 0.025M) and (B) acetonitrile at ambient column temperature with the last eluted compound in less than 17 min. The flow rate was maintained at 1/2.3 mLmin-1 with UV detection at 245/210 nm using time programming.
Conclusion: The optimized chromatographic method was used for stability, indicating assay of the cited drugs in the presence of their related compounds according to ICH Q2R1 guidelines.
中文翻译:

利用实验设计建立和优化 RP-HPLC 方法,用于估计存在相关化合物的两种选择性 5-羟色胺再摄取抑制剂/苯二氮卓类组合
背景:实验设计 (DOE) 被认为是识别影响变异的因素并通过调整这些因素来改善响应的最强大工具。在目前的工作中,DOE 用于建立一种创新、灵敏且精确的 HPLC 方法,用于在原料药和制剂中存在相关化合物的情况下同时测定草酸艾司西酞普兰、盐酸帕罗西汀半水合物和氯硝西泮。
方法:使用 Plackett-Burman 设计 (PBD) 筛选缓冲液摩尔浓度、梯度开始/结束时的有机改性剂百分比(乙腈含量)、梯度开始/结束时的流速、流动相的 pH 值和柱温) 模型。主效应图显示梯度开始/结束时的有机改性剂百分比和梯度开始时的流速是影响峰分辨率的统计学显着变量 (p<0.05)。然后使用 Box-Behnken 设计 (BBD) 作为优化模型,通过研究这三个因素的相互作用和二次效应,以最少的实验次数获得尽可能高的分辨率。最后,通过合意性函数可以达到预测峰分辨率的最佳条件。
结果:优化后,在 Agilent Zorbax SB C18(4.6×250mm,5μm)色谱柱上实现色谱分离,流动相梯度洗脱:(A)磷酸二氢钾(pH 2.7;0.025M)和(B)乙腈,室温最后洗脱的化合物在不到 17 分钟的时间内达到柱温。流速保持在 1/2.3 mLmin-1,使用时间编程在 245/210 nm 处进行 UV 检测。
结论:优化的色谱方法用于稳定性,表明根据 ICH Q2R1 指南,在相关化合物存在的情况下对引用的药物进行分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号