Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2021-12-22 , DOI: 10.1016/j.cherd.2021.12.033 Gabriele Bano 1 , Ranjit M. Dhenge 1 , Samir Diab 1 , Daniel J. Goodwin 1 , Lee Gorringe 1 , Misbah Ahmed 1 , Richard Elkes 1 , Simeone Zomer 1
|
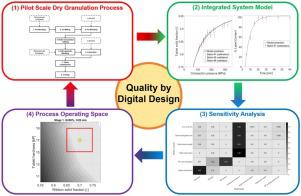
In industrial practice, the development of pharmaceutical dry granulation processes typically involves time- and resource-intensive multivariate experiments. These experiments are used to identify the set of operating conditions, their allowed ranges and chosen setpoints where the desired product quality and manufacturability criteria are met. The results are then used to define the control strategy for the manufacturing process to be included in the regulatory file.
In this study, we show how systems modelling can be used to streamline the development of an industrial dry granulation process for an immediate release tablet. We integrate existing and enhanced unit operation and product performance models with a Bayesian hierarchical model to predict the probability to meet the USP <711> dissolution test specifications, which represent the current standard in the pharmaceutical industry to demonstrate compliance with regulatory expectations. We then use global sensitivity analysis to: (i) generate multivariate process understanding on the relative impact of material properties and process parameters on product quality attributes; (ii) predict the set of operating conditions (i.e., the process operating space) that allows us to meet the USP <711> test specifications with a given probability, as well as pre-defined manufacturability criteria. We finally use the results obtained at point (ii) to design targeted experiments to verify the predicted setpoints and operating space. We show how the proposed framework has the potential to remove >60% of the experimental burden (and hence the consumption of active pharmaceutical ingredient) required for process development compared to standard experimental protocols.
中文翻译:

使用系统建模简化用于立即释放片剂的工业干法制粒工艺的开发
在工业实践中,药物干法制粒工艺的开发通常涉及时间和资源密集型多变量实验。这些实验用于确定一组操作条件、它们的允许范围以及满足所需产品质量和可制造性标准的选定设定点。然后将结果用于定义要包含在监管文件中的制造过程的控制策略。
在这项研究中,我们展示了如何使用系统建模来简化立即释放片剂的工业干法制粒工艺的开发。我们将现有和增强的单元操作和产品性能模型与贝叶斯分层模型相结合,以预测满足 USP <711> 溶出度测试规范的可能性,该规范代表了制药行业的当前标准,以证明符合监管预期。然后我们使用全局敏感性分析来:(i) 对材料特性和工艺参数对产品质量属性的相对影响产生多变量工艺理解;(ii) 预测一组操作条件(即进程操作空间) 使我们能够以给定的概率满足 USP <711> 测试规范以及预定义的可制造性标准。我们最终使用在点 (ii) 获得的结果来设计有针对性的实验,以验证预测的设定点和操作空间。我们展示了与标准实验方案相比,所提出的框架如何有可能消除工艺开发所需的 >60% 的实验负担(以及活性药物成分的消耗)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号