Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2021-12-18 , DOI: 10.1016/j.jtice.2021.104175 Chin-Wei Chang , Liang-Shin Wang , Nam Ngoc Pham , Chih-Che Shen , Mu-Nung Hsu , Nuong Thi Kieu Nguyen , Chia-Yi Yen , Mei-Wei Lin , Jih-Ru Hwu , Yu-Han Chang , Yu-Chen Hu
|
Background
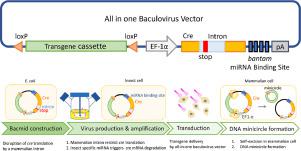
Insect baculovirus is a promising vector for gene delivery into mammalian cells. We developed a Cre/loxP-based hybrid baculovirus comprising two viruses: one expressing Cre recombinase and the other harboring the transgene flanked by two loxP sites. Co-transduction of mammalian cells with two viruses confers Cre expression, which excises the loxP-flanking cassette off baculovirus genome and catalyzes DNA minicircle formation, thereby prolonging transgene expression. Two separate baculoviruses avoid undesirable recombination and loss of transgene during virus production process, but reduces gene delivery efficiency and complicates applications.
Methods
To tackle this problem, we exploited synthetic biology strategy to control Cre expression and develop an all-in-one baculovirus harboring both cre and loxP-flanking transgene cassette. We controlled cre transcription with a mammalian EF-1α promoter, and regulated cre translation with an intron inserted within the coding region and bantam miRNA binding site at the 3′UTR.
Significant findings
The all-in-one baculovirus vector selectively conferred Cre/loxP-mediated recombination in mammalian cells, but not in E. coli and Sf-9 cells, hence circumventing transgene excision from the virus genome during gene cloning in E. coli and virus amplification in insect cells. The all-in-on vector enabled formation of DNA minicircle in mammalian cells at efficiencies exceeding 80% and implicated its potential for gene delivery. Our design may be expanded to other gene delivery systems that require two separate vectors and is beneficial to gene therapy applications.
中文翻译:

使用哺乳动物内含子和 miRNA 结合位点开发多合一杆状病毒载体的合成生物学方法
背景
昆虫杆状病毒是一种很有前景的载体,可用于将基因传递到哺乳动物细胞中。我们开发了一种基于 Cre/loxP 的混合杆状病毒,它包含两种病毒:一种表达 Cre 重组酶,另一种携带两侧有两个 loxP 位点的转基因。哺乳动物细胞与两种病毒的共转导赋予 Cre 表达,从杆状病毒基因组中切除 loxP 侧翼盒并催化 DNA 小环形成,从而延长转基因表达。两种单独的杆状病毒在病毒生产过程中避免了不需要的重组和转基因丢失,但降低了基因传递效率并使应用复杂化。
方法
为了解决这个问题,我们利用合成生物学策略来控制 Cre 表达并开发了一种包含cre和 loxP 侧翼转基因盒的多合一杆状病毒。我们用哺乳动物 EF-1α 启动子控制cre转录,并通过插入编码区和 3'UTR 的矮脚鸡 miRNA 结合位点的内含子调节cre翻译。
重要发现
多合一杆状病毒载体在哺乳动物细胞中选择性地赋予 Cre/loxP 介导的重组,但不在大肠杆菌和 Sf-9 细胞中,从而避免在大肠杆菌中进行基因克隆和病毒扩增期间从病毒基因组中切除转基因在昆虫细胞中。这种全方位载体能够以超过 80% 的效率在哺乳动物细胞中形成 DNA 小环,并暗示其具有基因传递的潜力。我们的设计可能会扩展到其他需要两个独立载体的基因传递系统,并有利于基因治疗应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号