Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2021-12-18 , DOI: 10.1016/j.jtice.2021.104168 Mohamed Shaker S. Adam 1, 2 , Omar M. El-Hady 2 , Mohamed M. Makhlouf 3 , Abrar Bayazeed 4 , Nashwa M. El-Metwaly 4, 5 , Ahmad Desoky M. Mohamad 2
|
Background
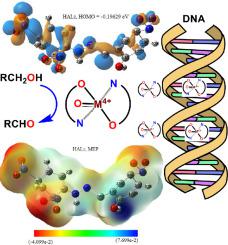
Based on the high coordination behavior of arylhydrazones, as pincer chelating agents, the purpose of this research is to study the synthesis, characterization and reactivity of two mononuclear oxy-vanadium (IV) and oxy- zirconium (IV) complexes (VO(ALz)2 and ZrO(ALz)2, respectively) of O,N-monobasic bidenate arylhydazone derivative (2-((2-(2,4-dinitrophenyl) hydrazineylidene)methyl)-4-nitrophenol, HALz).
Methods
Various spectroscopic methods were applied to characterize the chemical structures of the ligand and their O=M4+-complexes (NMR, UV-Vis., Mass and IR spectra). Their reactivity was estimated catalytically and biologically.
The significant findings
Here, the function of the oxy-highly charged metal ion of (O=M4+: V4+ and Zr4+-ions) on reactivity of their arylhydrazone complexes was reported catalytically and biologically. The differentiation in the catalytic features of VO(ALz)2 and ZrO(ALz)2 depends on the type of O=M4+ ion, which examined in the aerobic oxidation of benzyl alcohol using tert-butyl hydroperoxide (tBuOOH) under solvent free atmosphere at 90 °C (the optimized conditions). VO(ALz)2 exhibited little more progressed catalytic potential (2 h with 89% yield of benzaldehyde) over that of ZrO(ALz)2 (6 h with 82% yield of benzaldehyde). The electron-oxygen gain/loss of O=M4+ ion in its complex catalyst displayed the major role for progressing of its catalytic potential. The catalytic oxidation of secondary alcohols to their corresponded ketones was performed with VO(ALz)2 and ZrO(ALz)2 catalysts. A mechanistic pathway was proposed depending on via DFT approach and on spectroscopic aspects.
The biological action of HALz, VO(ALz)2 and ZrO(ALz)2 were studied within their binding action towards ctDNA (through UV–Visible spectroscopy and viscosity measuring) depending on the type of O=M4+ ion in their reagents. The interaction of HALz, VO(ALz)2 and ZrO(ALz)2 and was evaluated with the binding constant (2.85, 6.07 and 5.42 × 105 mol−1 dm3, respectively), which referred to their high reactivity towards ctDNA. In addition, the values of Gibbs’ free energy supported their ctNDA interaction (-31.11, -32.99 and -32.71 kJ mol−1, respectively). Both V4+ and Zr4+ ions in their complexes promoted their reactivity as antioxidant, antimicrobial and anticancer reagents more that their uncoordinated HALz ligand. The interaction with DNA-pockets was investigated through in-silico ways regarding pharmacophore query and MOE-docking module, to strengthen the biological screening. Considerably, the current MO-complexes with their effective antimicrobial, antioxidant, and cytotoxicity activity would be probably appraised for further medicinal and pharmaceutical applications.
中文翻译:

O,N-二齿芳腙配合物中氧钒 (IV) 和氧锆 (IV) 离子对其催化和生物潜力的影响, 计算机应用支持
背景
基于芳基腙作为钳形螯合剂的高配位行为,本研究的目的是研究两种单核氧钒(IV)和氧锆(IV)配合物(VO(ALz) O,N-二烯酸芳基腙衍生物(2-((2-(2,4-二硝基苯基)亚肼基)甲基)-4-硝基苯酚,HALz)的2和ZrO(ALz) 2 )。
方法
应用各种光谱方法来表征配体及其O=M 4+ -配合物的化学结构(NMR、UV-Vis.、质谱和IR光谱)。它们的反应性是通过催化和生物学估计的。
重大发现
在这里,以催化和生物学的方式报道了(O=M 4+ : V 4+和Zr 4+ -离子)的高氧金属离子对其芳基腙配合物反应性的作用。VO(ALz) 2和ZrO(ALz) 2催化特性的区别取决于O=M 4+离子的类型,在溶剂下使用叔丁基过氧化氢( t BuOOH)对苯甲醇的有氧氧化进行了检测90 °C 下的自由气氛(优化条件)。与 ZrO(ALz) 2相比, VO(ALz) 2表现出的催化潜力(2 小时,苯甲醛产率为 89%)稍有进步(6 小时,苯甲醛收率为 82%)。O=M 4+离子在其络合物催化剂中的电子-氧增益/损失显示出其催化潜力发展的主要作用。用VO(ALz) 2和ZrO(ALz) 2催化剂将仲醇催化氧化成相应的酮。根据通过DFT 方法和光谱方面提出了一种机械途径。
根据试剂中 O=M 4+离子的类型,研究了 HALz、VO(ALz) 2和 ZrO(ALz) 2对ct DNA 的结合作用(通过紫外-可见光谱和粘度测量)的生物学作用. HALz、VO(ALz) 2和 ZrO(ALz) 2的相互作用并用结合常数(分别为 2.85、6.07 和 5.42 × 10 5 mol -1 dm 3)进行评估,这表明它们对ct DNA具有高反应性. 此外,吉布斯自由能值支持它们的ct NDA 相互作用(-31.11、-32.99 和 -32.71 kJ mol -1, 分别)。复合物中的V 4+和Zr 4+离子都比它们的未配位HALz配体更能促进它们作为抗氧化剂、抗微生物和抗癌试剂的反应性。通过药效团查询和MOE对接模块的in-silico方式研究与DNA-pocket的相互作用,以加强生物筛选。值得注意的是,目前具有有效抗菌、抗氧化和细胞毒性活性的 MO 复合物可能会被评估用于进一步的医学和制药应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号