Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-12-17 , DOI: 10.1016/j.jcou.2021.101862 J. Arturo Mendoza-Nieto 1 , Héctor Martínez-Hernández 2 , Heriberto Pfeiffer 3 , J. Francisco Gómez-García 4
|
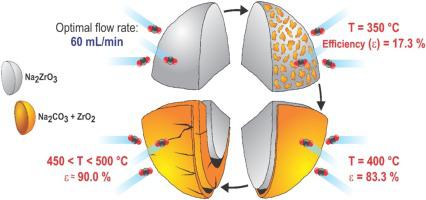
In this work, it is presented a kinetic study for CO2 capture on Na2ZrO3 as a function of temperature (250−550 °C) and CO2 flow rate (5, 60, and 120 mL/min). We discuss the relevance of a new kinetic model able to fit the experimental data and propose a consecutive reaction model for CO2 capture under low, moderate, and high CO2 flow rates. The proposed model considers that a Na2CO3–ZrO2 layer is formed at the particle surface level, and then, the capture process is later enhanced by Na+ ions diffusion, but its improvement depends entirely on the surface capture. This kinetic approach allowed a better fit for CO2 capture results, describing well the variations obtained as a function of the different flow rates tested between 250 and 550 °C. Thermogravimetric and kinetic data showed that the CO2 capture was benefited as the CO2 flow rate increased from 5 to 120 mL/min. However, high flow rates (60 and 120 mL/min) achieved similar capture efficiencies (Ɛ ≈ 90 %), only once the CO2 sorption-desorption equilibrium was reached. In addition, through the Eyring model analysis, the activated state energy was calculated, being within the range of 0.84 and 1.20 eV for the surface capture and between 0.89 and 1.02 eV for bulk capture (related to Na+ diffusion). In the final section, it was discussed the CO2 sorption-desorption process takes place at both, low temperature and flow rate, and how such a process inhibits or triggers high-temperature CO2 capture.
中文翻译:

锆酸钠 (Na2ZrO3) 捕获 CO2 的新动力学模型:不同流速下的分析
在这项工作中,提出了在 Na 2 ZrO 3上捕获CO 2的动力学研究,作为温度(250-550 °C)和 CO 2流速(5、60和 120 mL/min)的函数。我们讨论了能够拟合实验数据的新动力学模型的相关性,并提出了在低、中和高 CO 2流速下捕获CO 2的连续反应模型。所提出的模型认为在粒子表面水平形成了 Na 2 CO 3 –ZrO 2层,然后,Na +增强了捕获过程离子扩散,但其改进完全取决于表面捕获。这种动力学方法可以更好地拟合 CO 2捕获结果,很好地描述了作为在 250 到 550 °C 之间测试的不同流速的函数而获得的变化。热重和动力学数据表明,当 CO 2流速从 5 毫升/分钟增加到 120 毫升/分钟时,CO 2捕获受益。然而,高流速(60 和 120 mL/min)实现了相似的捕获效率(Ɛ ≈ 90 %),仅当 CO 2达到吸附-解吸平衡。此外,通过 Eyring 模型分析,计算出活化态能量,对于表面捕获在 0.84 和 1.20 eV 之间,对于体捕获(与 Na +扩散有关)在 0.89 和 1.02 eV 之间。在最后一节中,讨论了 CO 2吸附-解吸过程在低温和低流速下发生,以及这种过程如何抑制或触发高温 CO 2捕获。











































 京公网安备 11010802027423号
京公网安备 11010802027423号