Chemical Engineering Research and Design ( IF 3.9 ) Pub Date : 2021-12-15 , DOI: 10.1016/j.cherd.2021.12.018 Abraham Marmur 1
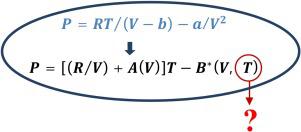
|
Many equations of state have been developed to improve the van der Waals equation from a quantitative point of view. The corrections to this equation are usually functions of the molar volume and the temperature, and they were developed by a sophisticated trial and error process. The present paper presents a methodology for creating equations of state based on mathematical principles. Equations of state can be viewed as solutions to a first-order partial differential equation that can be solved by the methods of characteristics. The characteristics in this case are isochores that appear to be straight lines to a very good approximation. This property of isochore linearity implies that the corrections to the van der Waals equation should be dependent only on the molar volume and not on temperature. This makes the development process much easier because it is necessary to elucidate the dependence on only one variable. As an example, a simple and very accurate equation of state is developed based on correction terms to the van der Waals equation that depend on the molar volume only.
中文翻译:

状态方程:数学发展方法的演示
已经开发了许多状态方程来从定量的角度改进范德瓦尔斯方程。该方程的修正通常是摩尔体积和温度的函数,它们是通过复杂的试错过程开发的。本文提出了一种基于数学原理创建状态方程的方法。状态方程可以看作是一阶偏微分方程的解,可以用特征方法求解。这种情况下的特征是等位点,它们看起来是非常近似的直线。等浓度线性的这种特性意味着对范德华方程的修正应仅取决于摩尔体积而不取决于温度。这使得开发过程更加容易,因为有必要阐明仅对一个变量的依赖性。例如,基于范德瓦尔斯方程的校正项开发了一个简单且非常准确的状态方程,该方程仅取决于摩尔体积。



























 京公网安备 11010802027423号
京公网安备 11010802027423号