Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-12-11 , DOI: 10.1016/j.jhep.2021.11.030 Ann-Lii Cheng , Shukui Qin , Masafumi Ikeda , Peter R. Galle , Michel Ducreux , Tae-You Kim , Ho Yeong Lim , Masatoshi Kudo , Valeriy Breder , Philippe Merle , Ahmed O. Kaseb , Daneng Li , Wendy Verret , Ning Ma , Alan Nicholas , Yifan Wang , Lindong Li , Andrew X. Zhu , Richard S. Finn
|
Background & Aims
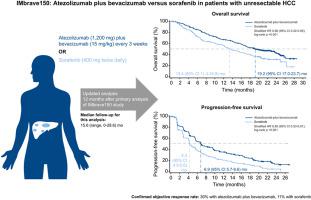
IMbrave150 demonstrated that atezolizumab plus bevacizumab led to significantly improved overall survival (OS) and progression-free survival (PFS) compared with sorafenib in patients with unresectable hepatocellular carcinoma at the primary analysis (after a median 8.6 months of follow-up). We present updated data after 12 months of additional follow-up.
Methods
Patients with systemic treatment-naive, unresectable hepatocellular carcinoma were randomized 2:1 to receive 1,200 mg atezolizumab plus 15 mg/kg bevacizumab intravenously every 3 weeks or 400 mg sorafenib orally twice daily in this open-label, phase III study. Co-primary endpoints were OS and PFS by independently assessed RECIST 1.1 in the intention-to-treat population. Secondary efficacy endpoints included objective response rates and exploratory subgroup efficacy analyses. This is a post hoc updated analysis of efficacy and safety.
Results
From March 15, 2018, to January 30, 2019, 501 patients (intention-to-treat population) were randomly allocated to receive atezolizumab plus bevacizumab (n = 336) or sorafenib (n = 165). On August 31, 2020, after a median 15.6 (range, 0-28.6) months of follow-up, the median OS was 19.2 months (95% CI 17.0–23.7) with atezolizumab plus bevacizumab and 13.4 months (95% CI 11.4–16.9) with sorafenib (hazard ratio [HR] 0.66; 95% CI 0.52-0.85; descriptive p <0.001). The median PFS was 6.9 (95% CI 5.7-8.6) and 4.3 (95% CI 4.0-5.6) months in the respective treatment groups (HR 0.65; 95% CI 0.53-0.81; descriptive p < 0.001). Treatment-related grade 3/4 adverse events occurred in 143 (43%) of 329 and 72 (46%) of 156 safety-evaluable patients in the respective groups, and treatment-related grade 5 events occurred in 6 (2%) and 1 (<1%) patients.
Conclusion
After longer follow-up, atezolizumab plus bevacizumab maintained clinically meaningful survival benefits over sorafenib and had a safety profile consistent with the primary analysis.
ClinicalTrials.gov identifier
NCT03434379.
Lay summary
The primary analysis of IMbrave150 showed that atezolizumab plus bevacizumab had significantly greater benefits than sorafenib in patients with advanced hepatocellular carcinoma, but survival data were not yet mature. At this updated analysis done 12 months later, median overall survival was 5.8 months longer with atezolizumab plus bevacizumab than sorafenib, and the severity profile of treatment-related side effects remained similar. These updated results confirm atezolizumab plus bevacizumab as the first-line standard of care for advanced hepatocellular carcinoma.
中文翻译:

来自 IMbrave150 的最新疗效和安全性数据:Atezolizumab 加贝伐珠单抗与索拉非尼治疗不可切除的肝细胞癌
背景与目标
IMbrave150 表明,在初步分析中(中位随访 8.6 个月后),与索拉非尼相比,atezolizumab 联合贝伐珠单抗可显着改善不可切除的肝细胞癌患者的总生存期 (OS) 和无进展生存期 (PFS)。我们在额外随访 12 个月后提供更新数据。
方法
在这项开放标签的 III 期研究中,未接受过全身治疗、无法切除的肝细胞癌患者按 2:1 随机分配接受每 3 周静脉注射 1,200 mg atezolizumab 加 15 mg/kg 贝伐珠单抗或每天两次口服 400 mg 索拉非尼。共同主要终点是在意向治疗人群中通过独立评估的 RECIST 1.1 得出的 OS 和 PFS。次要疗效终点包括客观反应率和探索性亚组疗效分析。这是对有效性和安全性的事后更新分析。
结果
从 2018 年 3 月 15 日到 2019 年 1 月 30 日,501 名患者(意向治疗人群)被随机分配接受阿替利珠单抗加贝伐珠单抗(n = 336)或索拉非尼(n = 165)。2020 年 8 月 31 日,经过中位 15.6(范围,0-28.6)个月的随访后,atezolizumab 加贝伐珠单抗的中位 OS 为 19.2 个月(95% CI 17.0-23.7)和 13.4 个月(95% CI 11.4- 16.9) 与索拉非尼(风险比 [HR] 0.66;95% CI 0.52-0.85;描述性p <0.001)。各治疗组的中位 PFS 分别为 6.9 (95% CI 5.7-8.6) 和 4.3 (95% CI 4.0-5.6) 个月(HR 0.65;95% CI 0.53-0.81;描述性p< 0.001)。治疗相关的 3/4 级不良事件发生在各组 329 名患者中的 143 名(43%)和 156 名安全性可评估患者中的 72 名(46%),治疗相关的 5 级事件发生在 6 名(2%)和 156 名患者中的 72 名(46%) 1 (<1%) 名患者。
结论
经过更长时间的随访,atezolizumab 联合贝伐珠单抗与索拉非尼相比,维持了具有临床意义的生存获益,并且安全性与初步分析一致。
ClinicalTrials.gov 标识符
NCT03434379。
外行总结
IMbrave150的初步分析表明,atezolizumab加贝伐珠单抗在晚期肝细胞癌患者中的获益明显大于索拉非尼,但生存数据尚未成熟。在 12 个月后进行的这项更新分析中,与索拉非尼相比,阿替利珠单抗加贝伐珠单抗的中位总生存期长 5.8 个月,并且治疗相关副作用的严重程度仍然相似。这些更新的结果证实阿替利珠单抗加贝伐珠单抗是晚期肝细胞癌的一线标准治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号