当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deep Eutectic Solvents Composed of Urea and New Salts of a Choline Family for Efficient Ammonia Absorption
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jced.1c00684 Olga V. Kazarina 1, 2 , Vira N. Agieienko 1 , Anton N. Petukhov 1, 3 , Andrey V. Vorotyntsev 1, 4 , Maria E. Atlaskina 1, 2 , Artem A. Atlaskin 5 , Sergey S. Kryuchkov 1, 3 , Atryom N. Markov 1, 3 , Alexander V. Nyuchev 6 , Ilya V. Vorotyntsev 5
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jced.1c00684 Olga V. Kazarina 1, 2 , Vira N. Agieienko 1 , Anton N. Petukhov 1, 3 , Andrey V. Vorotyntsev 1, 4 , Maria E. Atlaskina 1, 2 , Artem A. Atlaskin 5 , Sergey S. Kryuchkov 1, 3 , Atryom N. Markov 1, 3 , Alexander V. Nyuchev 6 , Ilya V. Vorotyntsev 5
Affiliation
|
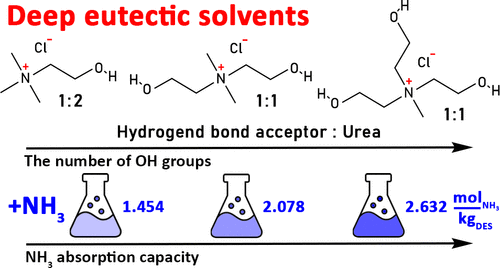
Two new deep eutectic solvents (DESs) composed of urea (U) and salts of a choline family such as dimethyl-di(2-hydroxyethyl)-ammonium chloride [Me2COH2N]Cl and methyl-tri(2-hydroxyethyl)-ammonium chloride [MeCOH3N]Cl mixed in a 1:1 molar ratio were prepared. Their densities, viscosities, refractive indices, and properties related to the ammonia absorption were thoroughly investigated. We found that the obtained DESs show an absorption capacity of 2.078 and 2.632 molNH3·kg–1DES for [Me2COH2N]Cl/U and [MeCOH3N]Cl/U, respectively, at 313.2 K and 101.3 kPa, which is approximately two times higher than for the choline chloride/urea (2:3) DES at the same conditions. Also, the obtained Henry’s law constants are of the same sequence; that is, they increase from [MeCOH3N]Cl/U to [Me2COH2N]Cl/U and finally to choline chloride/urea. The mentioned tendencies show good correlation with DES’s molar volume and its free volume, and also with the number of the CH2CH2OH substituents in a quaternary ammonium salt cation. This suggests that the gas capture occurs via the interactions (most likely H-bonding) between an NH3 molecule(s) and the hydroxyalkyl fragments. Indeed, the results of 1H NMR and FTIR spectroscopy confirm strong coupling between ammonia and hydroxyls leading to a distortion of the inherent DES structure. This finding agrees well with the fact that an NH3 dissolution process in the studied DESs is favored by an entropic contribution.
中文翻译:

由尿素和胆碱族新盐组成的深共晶溶剂用于高效吸收氨
两种新的低共熔溶剂 (DES),由尿素 (U) 和胆碱家族的盐组成,例如二甲基-二(2-羟乙基)-氯化铵 [Me 2 C OH 2 N]Cl 和甲基-三(2-羟乙基) )-氯化铵[MeC OH 3 N]Cl 以1:1 的摩尔比混合制备。对它们的密度、粘度、折射率和与氨吸收有关的特性进行了深入研究。我们发现所获得的DES对 [Me 2 C OH 2 N]Cl/U 和 [MeC OH 3的吸收能力分别为 2.078 和 2.632 mol NH3 ·kg –1 DESN]Cl/U 分别为 313.2 K 和 101.3 kPa,大约是相同条件下氯化胆碱/尿素 (2:3) DES 的两倍。另外,得到的亨利定律常数是同一个序列;也就是说,它们从 [MeC OH 3 N]Cl/U 增加到 [Me 2 C OH 2 N]Cl/U,最后增加到氯化胆碱/尿素。上述趋势与DES的摩尔体积及其自由体积以及季铵盐阳离子中的CH 2 CH 2 OH取代基的数量显示出良好的相关性。这表明气体捕获是通过 NH 3之间的相互作用(很可能是 H 键)发生的。分子和羟烷基片段。事实上,1 H NMR 和 FTIR 光谱的结果证实氨和羟基之间的强耦合导致固有 DES 结构的扭曲。这一发现与所研究的 DES 中的 NH 3溶解过程受到熵贡献的支持这一事实非常吻合。
更新日期:2022-01-13
中文翻译:

由尿素和胆碱族新盐组成的深共晶溶剂用于高效吸收氨
两种新的低共熔溶剂 (DES),由尿素 (U) 和胆碱家族的盐组成,例如二甲基-二(2-羟乙基)-氯化铵 [Me 2 C OH 2 N]Cl 和甲基-三(2-羟乙基) )-氯化铵[MeC OH 3 N]Cl 以1:1 的摩尔比混合制备。对它们的密度、粘度、折射率和与氨吸收有关的特性进行了深入研究。我们发现所获得的DES对 [Me 2 C OH 2 N]Cl/U 和 [MeC OH 3的吸收能力分别为 2.078 和 2.632 mol NH3 ·kg –1 DESN]Cl/U 分别为 313.2 K 和 101.3 kPa,大约是相同条件下氯化胆碱/尿素 (2:3) DES 的两倍。另外,得到的亨利定律常数是同一个序列;也就是说,它们从 [MeC OH 3 N]Cl/U 增加到 [Me 2 C OH 2 N]Cl/U,最后增加到氯化胆碱/尿素。上述趋势与DES的摩尔体积及其自由体积以及季铵盐阳离子中的CH 2 CH 2 OH取代基的数量显示出良好的相关性。这表明气体捕获是通过 NH 3之间的相互作用(很可能是 H 键)发生的。分子和羟烷基片段。事实上,1 H NMR 和 FTIR 光谱的结果证实氨和羟基之间的强耦合导致固有 DES 结构的扭曲。这一发现与所研究的 DES 中的 NH 3溶解过程受到熵贡献的支持这一事实非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号