Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2021-12-09 , DOI: 10.1016/j.jcmgh.2021.11.011 Zaichao Xu 1 , Li Zhao 1 , Youquan Zhong 1 , Chengliang Zhu 2 , Kaitao Zhao 1 , Yan Teng 1 , Xiaoming Cheng 3 , Qiang Chen 4 , Yuchen Xia 1
|
Background and Aims
The persistence of viral covalently closed circular DNA (cccDNA) is the major obstacle for antiviral treatment against hepatitis B virus (HBV). Basic and translational studies are largely hampered due to the lack of feasible small animal models to support HBV cccDNA formation. The aim of this study is to establish a novel mouse model harboring cccDNA.
Methods
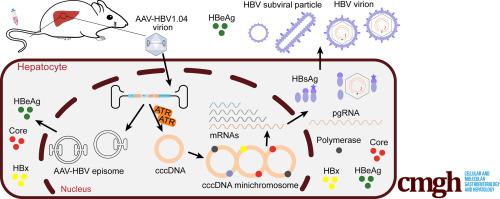
An adeno-associated virus (AAV) vector carrying a replication-deficient HBV1.04-fold genome (AAV-HBV1.04) was constructed. The linear HBV genome starts from nucleotide 403 and ends at 538, which results in the splitting of HBV surface and polymerase genes. Different HBV replication markers were evaluated for AAV-HBV1.04 plasmid–transfected cells, the AAV-HBV1.04 viral vector–transduced cells, and mice injected with the AAV-HBV1.04 viral vector.
Results
Compared with the previously reported AAV-HBV1.2 construct, direct transfection of AAV-HBV1.04 plasmid failed to produce hepatitis B surface antigen and progeny virus. Interestingly, AAV-HBV1.04 viral vector transduction could result in the formation of cccDNA and the production of all HBV replication markers in vitro and in vivo. The formation of cccDNA could be blocked by ATR (ataxia-telangiectasia and Rad3-related protein) inhibitors but not HBV reverse transcription inhibitor or capsid inhibitors. The AAV-HBV1.04 mouse supported long-term HBV replication and responded to antiviral treatments.
Conclusions
This AAV-HBV1.04 mouse model can support HBV cccDNA formation through ATR-mediated DNA damage response. The de novo formed cccDNA but not the parental AAV vector can lead to the production of hepatitis B surface antigen and HBV progeny. This model will provide a unique platform for studying HBV cccDNA and developing novel antivirals against HBV infection.
中文翻译:

一种携带乙型肝炎病毒共价闭合环状 DNA 的新型小鼠模型
背景和目标
病毒共价闭合环状 DNA (cccDNA) 的持续存在是乙型肝炎病毒 (HBV) 抗病毒治疗的主要障碍。由于缺乏可行的小动物模型来支持 HBV cccDNA 的形成,基础研究和转化研究在很大程度上受到了阻碍。本研究的目的是建立一种含有cccDNA的新型小鼠模型。
方法
构建了携带复制缺陷型HBV1.04倍基因组(AAV-HBV1.04)的腺相关病毒(AAV)载体。线性 HBV 基因组从核苷酸 403 开始,到 538 结束,这导致 HBV 表面和聚合酶基因的分裂。对 AAV-HBV1.04 质粒转染的细胞、AAV-HBV1.04 病毒载体转导的细胞和注射了 AAV-HBV1.04 病毒载体的小鼠评估了不同的 HBV 复制标志物。
结果
与先前报道的 AAV-HBV1.2 构建体相比,直接转染 AAV-HBV1.04 质粒未能产生乙型肝炎表面抗原和子代病毒。有趣的是,AAV-HBV1.04 病毒载体转导可导致 cccDNA 的形成和体外和体内所有 HBV 复制标志物的产生。cccDNA 的形成可以被 ATR(共济失调毛细血管扩张和 Rad3 相关蛋白)抑制剂阻断,但不能被 HBV 逆转录抑制剂或衣壳抑制剂阻断。AAV-HBV1.04 小鼠支持长期 HBV 复制并对抗病毒治疗有反应。
结论
这种 AAV-HBV1.04 小鼠模型可以通过 ATR 介导的 DNA 损伤反应支持 HBV cccDNA 的形成。从头形成的cccDNA 而不是亲本 AAV 载体可以导致乙型肝炎表面抗原和 HBV 后代的产生。该模型将为研究 HBV cccDNA 和开发针对 HBV 感染的新型抗病毒药物提供一个独特的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号