当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to γ-Carbolines: Synthesis of Isocryptolepine
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02026 Masahiro Akitake 1 , Shizuki Noda 1 , Kohei Miyoshi 1 , Motohiro Sonoda 1 , Shinji Tanimori 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02026 Masahiro Akitake 1 , Shizuki Noda 1 , Kohei Miyoshi 1 , Motohiro Sonoda 1 , Shinji Tanimori 1
Affiliation
|
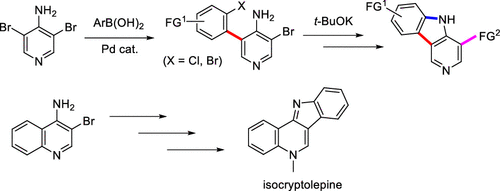
A new method to synthesize γ-carboline derivatives has been developed starting from 3,5-dibromo-4-pyridinamine by monoarylation using the Suzuki–Miyaura cross-coupling reaction followed by the base-mediated ring closure to pyrrole formation. Synthesis of a series of γ-carboline derivations from the 4-brominated γ-carboline 4a has been achieved by employing various coupling reactions and N-alkylations. This method has been applied for the synthesis of the antimalarial and anticancer natural product isocryptolepine. The photophysical properties of novel γ-carboline derivations are also reported.
中文翻译:

获取 γ-咔啉:异隐烯的合成
已经开发了一种合成 γ-咔啉衍生物的新方法,该方法从 3,5-二溴-4-吡啶胺开始,使用 Suzuki-Miyaura 交叉偶联反应进行单芳基化,然后通过碱基介导的闭环形成吡咯。通过采用各种偶联反应和 N-烷基化,已实现从 4-溴化 γ-咔啉4a合成一系列 γ-咔啉衍生物。该方法已应用于抗疟和抗癌天然产物异隐脑平的合成。还报道了新型 γ-咔啉衍生物的光物理性质。
更新日期:2021-12-17
中文翻译:

获取 γ-咔啉:异隐烯的合成
已经开发了一种合成 γ-咔啉衍生物的新方法,该方法从 3,5-二溴-4-吡啶胺开始,使用 Suzuki-Miyaura 交叉偶联反应进行单芳基化,然后通过碱基介导的闭环形成吡咯。通过采用各种偶联反应和 N-烷基化,已实现从 4-溴化 γ-咔啉4a合成一系列 γ-咔啉衍生物。该方法已应用于抗疟和抗癌天然产物异隐脑平的合成。还报道了新型 γ-咔啉衍生物的光物理性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号