当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoredox Catalyzed Radical Cascade Aroylation (Sulfonylation)/Cyclization Enables Access to Fused Indolo-pyridones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02335 De-Yong Yang 1 , Liang Liu 1 , Jia-Yi Gu 1 , Yan-Hong He 1 , Zhi Guan 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02335 De-Yong Yang 1 , Liang Liu 1 , Jia-Yi Gu 1 , Yan-Hong He 1 , Zhi Guan 1
Affiliation
|
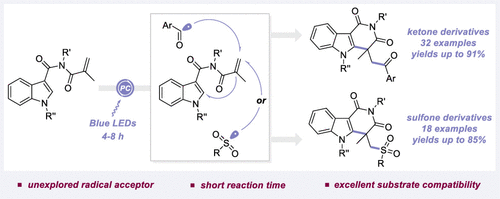
A visible-light-initiated radical cascade reaction toward the synthesis of structurally diverse fused Indolo-pyridones is described. The reaction involves the addition of aroyl or sulfonyl radicals to N-alkyl-acryloyl-1H-indole-3-carboxamides, cyclization, and oxidative aromatization. This telescoped method circumvents lengthy prefunctionalization steps of radical precursors, which is further underpinned by the superior compatibility with a series of C-centered radicals, allowing the rapid and facile construction of numerous valuable architectures.
中文翻译:

Photoredox 催化的自由基级联芳酰化(磺酰化)/环化能够获得稠合的吲哚-吡啶酮
描述了合成结构多样的稠合吲哚-吡啶酮的可见光引发的自由基级联反应。该反应包括将芳酰基或磺酰基加入N-烷基-丙烯酰基-1 H-吲哚-3-甲酰胺、环化和氧化芳构化。这种伸缩方法避免了自由基前体冗长的预官能化步骤,这进一步得到了与一系列 C 中心自由基的卓越兼容性的支持,从而可以快速轻松地构建许多有价值的架构。
更新日期:2021-12-17
中文翻译:

Photoredox 催化的自由基级联芳酰化(磺酰化)/环化能够获得稠合的吲哚-吡啶酮
描述了合成结构多样的稠合吲哚-吡啶酮的可见光引发的自由基级联反应。该反应包括将芳酰基或磺酰基加入N-烷基-丙烯酰基-1 H-吲哚-3-甲酰胺、环化和氧化芳构化。这种伸缩方法避免了自由基前体冗长的预官能化步骤,这进一步得到了与一系列 C 中心自由基的卓越兼容性的支持,从而可以快速轻松地构建许多有价值的架构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号