当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric α-Functionalization of 2-Alkyl Azaarenes: Synthesis of Tertiary Fluorides Having Vicinal Stereogenic Centers
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.orglett.1c03626 Arko Das 1 , Harshit Joshi 1 , Vinod K Singh 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.orglett.1c03626 Arko Das 1 , Harshit Joshi 1 , Vinod K Singh 1
Affiliation
|
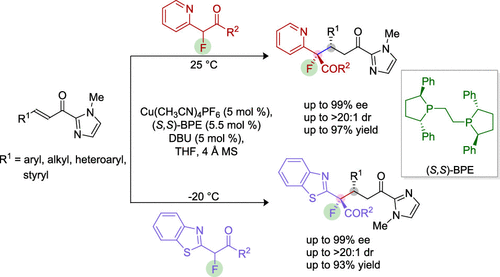
An enantioselective approach for synthesizing fluorinated azaarenes containing vicinal quaternary–tertiary stereocenters is summarized. The chiral copper(I)–phosphine complex binds with the azaarenes followed by Michael addition to unsaturated acyl imidazoles, resulting in α-functionalized products with an excellent level of enantioselectivities (up to 99%), diastereoselectivities (>20:1), and yields (up to 97%). Furthermore, post-functionalization of the acyl imidazole part has also been demonstrated.
中文翻译:

2-烷基氮杂芳烃的不对称α-功能化:具有邻位立体中心的叔氟化物的合成
总结了一种合成含有邻位季-叔立体中心的氟化氮杂芳烃的对映选择性方法。手性铜 (I)-膦络合物与氮杂芳烃结合,然后迈克尔加成不饱和酰基咪唑,从而产生具有优异水平对映选择性(高达 99%)、非对映选择性(>20:1)和产量(高达 97%)。此外,还证明了酰基咪唑部分的后官能化。
更新日期:2021-12-17
中文翻译:

2-烷基氮杂芳烃的不对称α-功能化:具有邻位立体中心的叔氟化物的合成
总结了一种合成含有邻位季-叔立体中心的氟化氮杂芳烃的对映选择性方法。手性铜 (I)-膦络合物与氮杂芳烃结合,然后迈克尔加成不饱和酰基咪唑,从而产生具有优异水平对映选择性(高达 99%)、非对映选择性(>20:1)和产量(高达 97%)。此外,还证明了酰基咪唑部分的后官能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号