当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
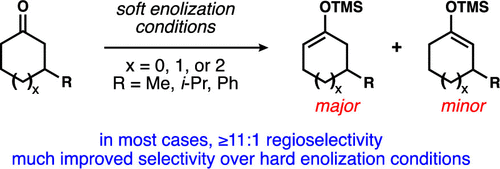
Soft Enolization of 3-Substituted Cycloalkanones Exhibits Significantly Improved Regiocontrol vs Hard Enolization Conditions
Organic Letters ( IF 5.2 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.orglett.1c03844 Natalie C Dwulet 1 , Vincenzo Ramella 1 , Christopher D Vanderwal 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.orglett.1c03844 Natalie C Dwulet 1 , Vincenzo Ramella 1 , Christopher D Vanderwal 1, 2
Affiliation
|
Soft enolization conditions are revealed to be markedly better than the typically applied hard enolization protocols for regioselective enoxysilane formation from unsymmetrical 3-substituted cycloalkanones. Five-, six-, and seven-membered cycloalkanones each with 3-methyl, 3-isopropyl, or 3-phenyl substituents were investigated, and in all but one case, regioselectivities were ≥11:1 for enolization away from the substituent. These results are complementary to the regiospecific enoxysilane formation derived from cycloalkenone conjugate addition/enolate silylation.
中文翻译:

3-取代环烷酮的软烯醇化表现出显着改善的区域控制与硬烯醇化条件
对于从不对称的 3-取代环烷酮形成区域选择性烯氧基硅烷,软烯醇化条件显着优于通常应用的硬烯醇化方案。研究了分别具有 3-甲基、3-异丙基或 3-苯基取代基的五元、六元和七元环烷酮,在除一种情况外的所有情况下,远离取代基的烯醇化区域选择性均≥11:1。这些结果与源自环烯酮共轭加成/烯醇化甲硅烷基化的区域特异性烯氧基硅烷形成互补。
更新日期:2021-12-17
中文翻译:

3-取代环烷酮的软烯醇化表现出显着改善的区域控制与硬烯醇化条件
对于从不对称的 3-取代环烷酮形成区域选择性烯氧基硅烷,软烯醇化条件显着优于通常应用的硬烯醇化方案。研究了分别具有 3-甲基、3-异丙基或 3-苯基取代基的五元、六元和七元环烷酮,在除一种情况外的所有情况下,远离取代基的烯醇化区域选择性均≥11:1。这些结果与源自环烯酮共轭加成/烯醇化甲硅烷基化的区域特异性烯氧基硅烷形成互补。



























 京公网安备 11010802027423号
京公网安备 11010802027423号